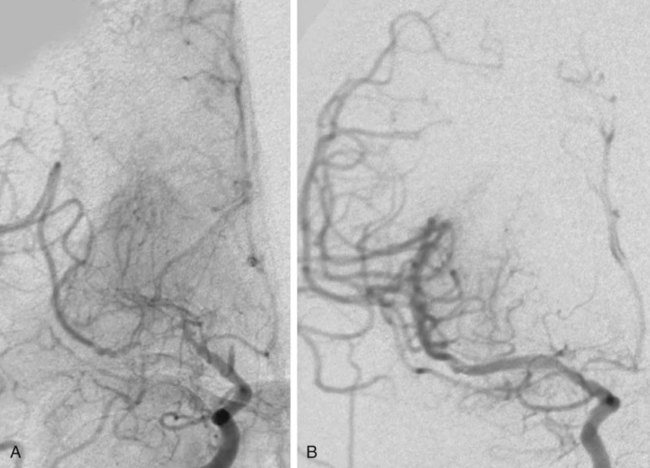
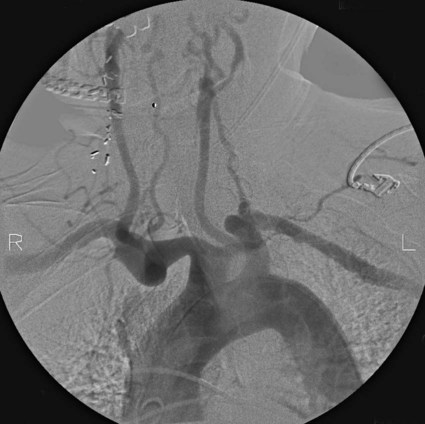
Chronic cerebral ischemia may be the result of extracranial carotid or vertebral stenosis and/or intracranial arterial stenoses. This chapter is going to focus on intracranial arterial stenoses as well as extracranial vertebral artery stenosis. For an in-depth discussion of extracranial carotid stenosis, please refer to Chapter 91, Carotid Revascularization. Intracranial atherosclerosis is estimated to be the underlying cause of 10% of all ischemic strokes1,2 and carries a first-year ipsilateral stroke rate of at least 11%.3 Twenty percent of all ischemic strokes involve the posterior circulation, and the most common area of vascular stenosis or occlusion in the setting of a posterior circulation stroke is the extracranial vertebral artery.4 Given these strong associations with stroke, intracranial atherosclerotic disease and extracranial vertebral artery atherosclerotic disease have become prime targets for endovascular treatment. The goal of intracerebral artery and extracranial vertebral endovascular revascularization in the setting of atherosclerotic stenosis is ipsilateral stroke prevention. Given the benign nature of asymptomatic intracranial atherosclerotic disease, asymptomatic patients with intracranial atherosclerotic narrowing should not be offered endovascular treatment.5 Regarding treatment of symptomatic intracranial atherosclerosis, it is generally believed that endovascular treatment should only be considered in patients who remain symptomatic despite maximal medical therapy. The rationale leading up to this conclusion, including the preliminary results of the SAMMPRIS study (Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis), are discussed in the following paragraphs. The utility of best medical therapy alone in the treatment of intracranial atherosclerotic disease was evaluated in the Warfarin-Aspirin Symptomatic Intra-cranial Disease Trial (WASID).3 In this study, patients with a history of stroke or transient ischemic attack (TIA) caused by an angiographically confirmed 50% to 99% intracranial artery stenosis were randomly assigned to receive aspirin (1300 mg; n = 280) or warfarin (target international normalized ratio 2.0 to 3.0; n = 289). The study was prematurely terminated because of higher death, major hemorrhage, and myocardial infarction rates in the warfarin group, without any primary endpoint benefit for either treatment (stroke/brain hemorrhage/vascular death other than stroke, 22.1% for aspirin vs. 21.8% for warfarin). Perhaps most importantly, this study illustrated the high morbidity and lack of effective medical therapy for moderate to severe intracranial atherosclerotic stenosis at that time. The overall 1- and 2-year rates of ischemic stroke in the territory of the stenosed artery were 11% and 14%, respectively. Factors associated with increased stroke risk included stenosis greater than 70%, recent symptoms, and female gender.6 The risk of a recent index event (TIA or stroke) and the linear increase in stroke risk associated with the degree of stenosis is analogous to the risk stratification observed in cervical carotid artery disease, which is estimated at 22% for severe symptomatic stenosis. Endovascular stenting is another treatment option that has been studied for the prevention of recurrent stroke or TIA in patients with intracranial atherosclerotic disease. However, evaluation of the efficacy of intracranial angioplasty and stenting is limited by factors such as rapidly evolving technology, the wide variation in endovascular techniques, and the overall lack of controlled data in the literature. Outcome after endovascular treatment for chronic cerebral ischemia is generally measured by technical success, incidence of restenosis, and early and long-term ipsilateral stroke rates. Technical success for intracerebral arterial and extracranial vertebral artery disease, defined as residual stenosis post procedure of less than 50%, is on the order of 95%.7–10 However, restenosis rates are high, with the Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) study8 reporting a 43% restenosis rate over a mean follow-up period of 16 months for extracranial vertebral artery stenting and a 32.4% restenosis rate for intracranial stenting. In estimating the overall risk of stroke and death associated with intracranial angioplasty with or without stenting, a Cochrane systematic review of 79 publications calculated the pooled periprocedural rate of stroke or death at 9.5%.11 The SSYLVIA study,8 a nonrandomized prospective protocol evaluating the feasibility of the Neurolink System (Guidant Corp., Indianapolis, Ind.) for vertebral and intracranial artery stenosis, included patients with 50% or greater stenosis by angiography with a recent history of stroke (60.7%) or TIA (39.3%). Failure of medical therapy before endovascular intervention was not a prerequisite for this study. Of the 61 patients included, 43 underwent treatment for symptomatic intracranial stenosis (15 internal carotid, 5 middle cerebral, 1 posterior cerebral, 17 basilar, 5 vertebral), and 18 underwent treatment for extracranial vertebral artery stenosis (6 ostial lesions and 12 lesions proximal to the posterior inferior cerebellar artery). Successful stenting with residual stenosis of less than 50% was attained in 95% (58/61 patients). However, as noted, restenosis rates were high: 12/37 or 32.4% for the intracranial arteries and 6/14 or 42.9% for the extracranial vertebrals. Among the 18 patients with restenosis, 7 (39%) were symptomatic. Strokes occurred in 6.6% of patients within 30 days and in 7.3% between 30 days and 1 year, and there were no deaths. In the prospective multicenter Wingspan trial,7 45 patients with symptomatic medically refractory intracranial stenosis of more than 50% were treated with angioplasty and Wingspan stent placement (Fig. 93-1). Technical success, defined as residual postprocedure stenosis of less than 50%, was achieved in 100% of cases. The incidence of restenosis was only 7.5%, much lower than in the SSYLVIA study, and the ipsilateral 30-day and 6-month combined stroke and death rates were 4.4% and 7.1%, respectively. The only prospective randomized study on the topic of intracranial angioplasty and stenting is the SAMMPRIS trial, which compared aggressive medical management alone to intracranial stenting plus aggressive medical management for the prevention of stroke in patients with symptomatic severe intracranial stenosis (70%-99%) of a major artery (middle cerebral, carotid, vertebral, or basilar arteries). In the context of the study, aggressive medical management consisted of aspirin 325 mg/day for the entire follow-up, clopidogrel 75 mg/day for 90 days after enrollment, intensive management of vascular risk factors (systolic blood pressure < 140 mmHg, [<130 mmHg if diabetic], low-density lipoprotein [LDL] levels below 70 mg/dL), and lifestyle modification programs for all study patients. Recruitment was halted after 451 (59%) of the planned 764 patients were enrolled, owing to the higher risk of stroke and death detected in the stented group (14%) compared to the aggressive medical management alone group (5.8%) in the first 30 days of enrollment.12 In the period beyond 30 days, the risk of stroke and death appear to be similar in the two groups, although most of the patients have not been followed for 1 year.12 The preliminary results of the SAMMPRIS trial illustrate both the significant benefit of aggressive medical management to treat severe symptomatic intracranial stenosis, and the important risks of endovascular treatment in this patient population. In summary, the results of the cited studies support considering endovascular treatment in patients with intracranial atherosclerotic disease only if they remain symptomatic despite maximal aggressive medical therapy. Intracranial angioplasty and stenting can also be indicated in a number of conditions besides atherosclerotic disease, although there is an overall paucity of data regarding these entities. Patients with symptomatic vertebral or intracerebral artery traumatic or idiopathic dissection who have failed adequate medical therapy may be considered for angioplasty and stent placement. Additionally, patients with nonatherosclerotic stenosis secondary to Takayasu arteritis, moyamoya disease, and connective tissue diseases such as fibromuscular dysplasia may benefit from angioplasty and possible stenting, although recent evidence in the case of moyamoya disease has shown that although such intervention may temporarily improve brain perfusion, angioplasty/stenting does not appear to provide long-term prevention against ischemic events in these patients.13 Furthermore, the authors’ own experience has shown high restenosis rates when angioplasty and stenting are performed in cases of arteritis. Relative clinical contraindications to endovascular management of chronic cerebral ischemia are related to the risk of cerebral angiography and include severe contrast allergy and chronic renal insufficiency. Both of these may be managed with appropriate premedication. Administration of diphenhydramine (50 mg, 1 hour preoperatively) and prednisone (50 mg at 24, 12, and 1 hour preoperatively) is effective contrast allergy prophylaxis. Hydration with intravenous sodium bicarbonate solution and oral administration of N-acetylcysteine before and after contrast agent administration has demonstrated efficacy in preventing contrast nephropathy.14,15 Other relative clinical contraindications are related to the need for combination antiplatelet therapy for at least 4 weeks after stenting. These include allergy or intolerance (excessive bruising) to aspirin and clopidogrel therapy. The requirement for combination antiplatelet therapy, intended to prevent platelet aggregation, embolization, and stent thrombosis until stent endothelialization, also makes the urgent need for a major surgical procedure a relative contraindication for stenting. Relative anatomic contraindications for stenting procedures are usually manageable by experienced operators when present individually. However, as the number of anatomic difficulties accrues, the procedure becomes significantly higher risk, making medical management preferable to the endovascular approach. Anatomic relative contraindications include severe tortuosity, calcification, or atherosclerotic disease of the aortic arch and origins of the great vessels (Fig. 93-2). These changes make endovascular access difficult and significantly raise the risk of thromboembolic complications. Great-vessel origin stenosis is manageable by angioplasty and stenting concurrently with treatment of the target vessel. Severe tortuosity and acute takeoff of the common carotid artery and severe tortuosity of the internal carotid artery make access for a guide catheter or sheath and delivery of angioplasty balloon and stent challenging. Other anatomic considerations, including tandem stenoses, unruptured intracranial aneurysms, and arteriovenous malformations, are not contraindications to endovascular therapy. Poorly controlled hypertension and a large recent infarct raise the risk of post-revascularization intracerebral hemorrhage from reperfusion injury and hyperperfusion syndrome. Postponing revascularization therapy (endovascular or surgical) 10 to 14 days after a moderate to large stroke and careful postoperative blood pressure control can effectively minimize these risks. Intensive medical therapy, including combination antiplatelet and high-dose statin therapy, in the first weeks after a stroke may also facilitate plaque stabilization, lowering the thromboembolic risk associated with the endovascular approach.
Endovascular Management of Chronic Cerebral Ischemia
Indications
Contraindications
Radiology Key
Fastest Radiology Insight Engine