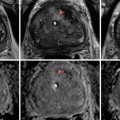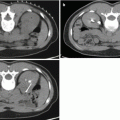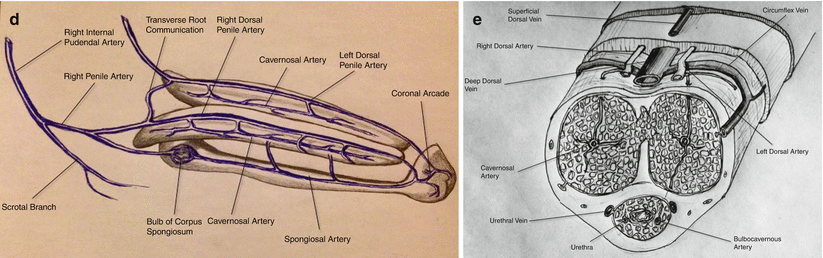
Fig. 25.1
(a): Arrow: Catheter tip in the left internal iliac artery demonstrating a selective digital subtraction angiography (DSA) of left internal iliac artery anatomy. A: Lateral sacral artery. B: Internal pudendal artery. C: Superior gluteal artery. D: Obturator artery. E: Inferior gluteal artery. (b) Selective DSA of normal internal pudendal artery anatomy. A: Internal pudendal artery. B: Inferior rectal branches. C: Penile artery. D: Perineal-scrotal artery. (c) Selective DSA of the internal pudendal artery demonstrating more distal branches. A: Internal pudendal artery. B: Inferior rectal arteries. C: Perineal-scrotal artery. D: Penile artery. E: Dorsal artery of the penis. F: Cavernosal artery. (d) Schematic drawing of the arterial anatomy supplying the penis. (e) Schematic drawing of the cross-sectional vascular anatomy of the penis
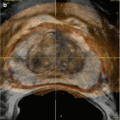
Venous drainage of the cavernous spaces is via three sets of veins (Fig. 25.2). The superficial dorsal vein drains the skin and subcutaneous tissues of the penis, ultimately draining into the superficial external pudendal vein. The deep dorsal vein drains the glans penis, the corpus spongiosum, and part of the corpora cavernosa. The cavernous veins are a venous plexus that drain the corpora and join the deep dorsal vein of the penis, which drains into the prostatic venous plexus.
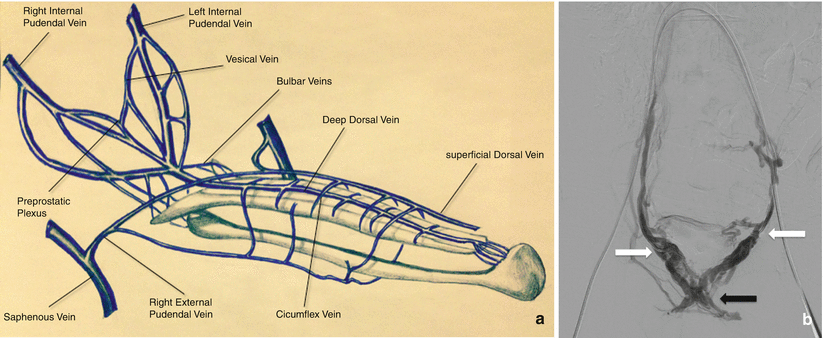

Fig. 25.2
(a) Schematic drawing of the venous drainage of the penis. (b) Venogram depicting the prostatic venous plexus. Access was obtained through the bilateral femoral veins. The catheters are seen extending through the contralateral internal iliac veins, with bilateral catheter tips at the junction of the internal pudendal veins/prostatic plexus (white arrows). Contrast is seen refluxing into the prostatic plexus (black arrow)
Erection is a neurovascular event. During the flaccid state, blood flow to the cavernous spaces is minimal due to vasoconstriction of the vasculature and shunting of blood flow away from the cavernous spaces. In response to sexual arousal, the parasympathetic nerves innervating the vascular smooth muscle of the deep arteries release nitric oxide, initiating a cascade that results in muscular relaxation. The vasodilation allows blood to flow into the cavernous spaces to induce engorgement and erection. This also has the effect of increasing pressure on the veins in the penis, reducing venous drainage.
Priapism
Priapism is a persistent erection of the penis that occurs hours beyond or in the absence of sexual stimulation [1]. Typically, priapism only affects the corpora cavernosa and is related to differences in the arterial inflow and venous outflow. Classically, two forms of priapism have been described—low flow (veno-occlusive, ischemic) and high flow (arterial, non-ischemic)—both of which differ in the etiologic pathophysiology and the available treatment options.
Low-Flow Priapism
Low-flow (veno-occlusive, ischemic) priapism is the most common type and is caused by impaired outflow from the corpora cavernosa. This leads to painful engorgement of the corpora cavernosa and subsequent ischemia of a cavernous tissue. This form of priapism is considered a form of compartment syndrome and therefore a urological emergency due to the potential for permanent damage to penile tissue and high frequency of erectile dysfunction if left untreated. The duration of priapism is the most important predictor of the development of subsequent erectile function in the setting of low-flow priapism [2]. Low-flow priapism can be associated with sickle cell disease, hematological malignancies, or medications; however, it most commonly is idiopathic in etiology [3]. Treatment of this form of priapism involves decompression of the corpora cavernosa via needle aspiration, which allows recovery of intracorporeal blood circulation. If this fails, direct injection of sympathomimetic agents is recommended prior to initiating surgical intervention [1]. There is little or no role of endovascular therapy in the management of low-flow priapism.
High-Flow Priapism
Classification and Pathophysiology
High-flow priapism, also called nonischemic or arterial priapism, is a rare condition that usually results from perineal or penile trauma, which leads to arteriocavernous fistula formation. Blood flows from one of the terminal branches of the internal pudendal artery—usually the cavernosal artery—into the lacunar spaces of the corpora cavernosa. The lacunar endothelium is then exposed to oxygenated blood with high velocity and turbulent flow causing shearing forces, which stimulate the release of nitric oxide. This results in arterial and trabecular dilatation throughout the corpora cavernosa [4, 5].
High-flow priapism can develop immediately following trauma or may not develop for a number of days following the inciting event. Arterial priapism is not a medical emergency because venous outflow remains intact and therefore the penis is not ischemic. The priapism can therefore persist for long periods of time without permanent damage of penile tissues [6].
Presentation
Clinically, a patient with high-flow priapism typically reports an erection that is not associated with pain and is frequently intermittent or not fully rigid. Typically there is a delay between the injury and the development of the priapism that may be up to a few days or longer. On physical examination, the corpora are tumescent but not fully rigid (in contrast to low-flow priapism), and there may be signs of perineal or penile trauma.
Diagnostic Evaluation
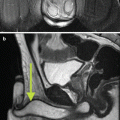
Needle aspiration, while not utilized in the treatment of high-flow priapism, can have a diagnostic role as one of the initial steps in determining if the priapism can be characterized as high or low flow. Diagnostic evaluation can also utilize ultrasound, which will show hypoechoic regions in the region of the arterial-sinusoidal fistula as well as direct visualization of the fistula on color Doppler imaging [4, 7]. Both computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have also been demonstrated as an effective tool for displaying penile arterial and venous anatomy and localizing the site of the fistula [8, 9].
Management
The American Urological Association states that the majority of arterial priapism cases resolve spontaneously and recommend conservative management as the first-line treatment for this form of priapism [1]. However, some studies suggest a low rate of resolution with conservative management [10] or only temporary resolution with conservative options, including ultrasonographic compression or intracavernous injection of alpha-adrenergic agents [11]. Many centers now manage this form of priapism by performing radiologic embolization of the feeding artery (Fig. 25.3). This technique was first described in 1977 utilizing autologous clot [12]. Since that time a number of studies have been published describing the technique with a variety of embolic agents, including Gelfoam, PVA, microcoils, and NBCA [10, 13, 14]. Although rare cases of bilateral fistulas have been described, unilateral embolization is recommended to avoid the risk of gangrene and erectile dysfunction. If bilateral embolization is necessary, absorbable embolic materials are preferred.
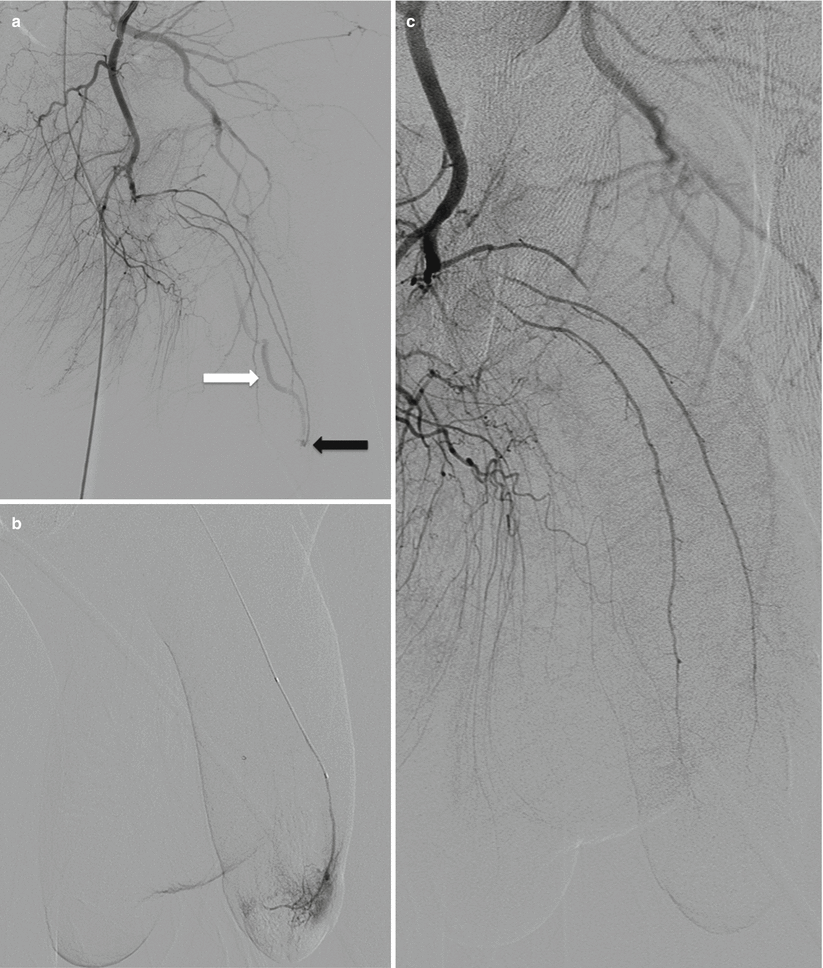

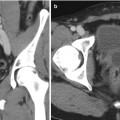
Fig. 25.3
(a) Selective catheterization of the left internal pudendal artery demonstrates early filling of the deep dorsal vein of the penis (white arrow) via an AV fistula in the distal aspect of the penis (black arrow). (b) More distal catheterization demonstrates the AV fistula with contrast filling the lacunar spaces of the distal corpora. (c) Control angiogram after embolization with Gelfoam demonstrates no visualization of the AV fistula. Patient’s intermittent priapism resolved within 2 weeks following the procedure
Overall clinical success rates with embolization are high, with resolution of the priapism seen within 24 h following the procedure. Recurrence rates ranging from 7 to 27 % have been reported after a single embolization treatment [15], also usually seen within the first 24 h following the procedure.
Erectile Dysfunction
Erectile dysfunction is the inability to achieve an erection or maintain an erection sufficient for satisfactory sexual performance [16]. The overall prevalence in the US male population over the age of 20 is approximately 18 %. The incidence increases with age, with approximately 70.25 % of men aged 70 years or older affected [17]. It is particularly associated with cardiovascular risk factors, including diabetes mellitus, hypertension, vascular disease, and dyslipidemia.
The AUA recommends that the initial evaluation of ED includes a complete medical, sexual, and psychosocial history [16]. The international index of erectile function (IIEF) is a questionnaire that has been developed to objectively assess erectile function [18]. The first-line therapy includes lifestyle and pharmacotherapy modification, as well as oral therapy with phosphodiesterase-5 inhibitors [19].
Further diagnostic testing is performed in a case-by-case manner and is based on the findings found during routine diagnostic workup. Because normal sexual function requires interactions among vascular, neurogenic, hormonal, and psychological systems, ED can result from the dysfunction of any one of these systems. Once psychogenic, neurogenic, and hormonal factors have been eliminated as possible causes, the focus shifts to an investigation of possible vasculogenic factors. Specific vascular causes of ED may be related to veno-occlusive dysfunction (VOD) or arterial insufficiency, both of which may be amenable to endovascular therapy.
Veno-Occlusive Dysfunction
With veno-occlusive dysfunction, incomplete relaxation of the corporeal smooth muscles causes incomplete resistance to outflow of blood from the corpora (previously termed “venous leakage”) and thus incomplete erection [20]. Patients with VOD often fare poorly with oral pharmacotherapy and frequently require long-term intracavernosal injection therapy [21]. Abnormalities of the venous system in erection can be diagnosed by cavernosometry, and the site of the venous “leakage” can be assessed with cavernosography. Both techniques are generally employed in young men who are already diagnosed with ED to evaluate for VOD.
In traditional cavernosography, two intracavernosal 19- to 21-gauge butterfly needles are placed dorsolaterally in each corpus cavernosum, approximately halfway down the shaft. Contrast medium diluted at approximately 1:4 with heparinized saline is slowly infused through one of the needles at a rate starting at 40 ml/min, with the rate slowly increased over time. Only one side needs to be injected because the septum between the corpora cavernosa is fenestrated. The contrast medium will be seen filling both corpora and the superficial and deep veins draining the pelvis. The second catheter is attached to a pressure transducer to record intracavernous pressure change. The flow rates needed to induce and maintain an erection are recorded [22].
In normal patients, infusion of approximately 80–120 mL of fluid will produce tumescence and finally an erection, with erection maintained at flow rates between 15 and 50 mL/min. Intracavernous pressure will rise to at least 80 mmHg for the duration of the erection. Veno-occlusive dysfunction is characterized by the absence of erection without significant increase of the intracavernous pressure. In many patients with VOD, much higher flow rates are needed to induce and subsequently maintain an erection [23].
Dynamic infusion cavernosometry and cavernosography (DICC) is a more comprehensive technique to evaluate the hemodynamic function of the penis. Unlike conventional cavernosography, DICC is performed after the intracavernosal injection of vasoactive agents, which lead to a pharmacologically induced erection, with subsequent provocative tests to determine the ability of the corpora cavernosa to optimally increase their venous outflow resistance. Systolic occlusion pressures in the left and right cavernosal arteries are also measured, which can help identify arterial occlusion or stenosis in the cavernous arterial bed [24].
Historically, erectile dysfunction caused by veno-occlusive dysfunction was treated by surgical ligation of the deep dorsal vein. Since that time, other surgical approaches have been described that include venous stripping or penile vascularization [25]. Subsequent research has investigated the role of interventional embolization of the deep dorsal vein of the penis in the treatment of erectile dysfunction caused by veno-occlusive dysfunction [20, 21, 26–30].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree