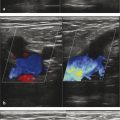
Percutaneous endovascular thermal ablation (EVTA) for varicose veins has been proven to achieve results comparable to surgical venous stripping with less associated periprocedural morbidity and faster recovery, attractive features that allow patients to be treated in the outpatient setting. Its main advantages are that it allows for the exclusive use of local anesthetic, is minimally invasive, and is performed in the outpatient procedure setting with immediate patient discharge and ambulation. Choice of treatment for varicose veins depends on the presence or absence of venous reflux. There are several therapies available when reflux is not detected: sclerotherapy, transdermal laser treatment, ablation with echosclerotherapy, and ambulatory phlebectomy. 1, 2, 3
When reflux is diagnosed, the classically performed intervention is saphenofemoral junction (SFJ) ligation with saphenous venous stripping. However, with the advent of ultrasound (US) and minimally invasive techniques resulting in a better understanding of anatomy and physiopathology of the venous disease, this approach has been increasingly replaced by EVTA under US guidance. 4, 5
Endovascular techniques for the treatment of varicose veins have emerged as a safe and effective alternative to traditional surgical therapy. 5 Recent clinical trials have shown equivalent long-term results compared to surgical vein stripping. 4, 5 Practitioners involved in the care of these patients should have a thorough understanding of imaging aspects of venous insufficiency, as it relates to pre- and postprocedure patient evaluation, as well as to the performance of image-guided intervention.
6.2 Physics of Thermal Ablation
There are two different options of energy for thermal ablation of incompetent veins: radiofrequency ablation (RFA) or endovenous laser therapy (EVLT). RFA employs heat generated from high-frequency alternating current (AC), in the range of 350 to 500 kHz. In contrast to low-frequency AC or pulses of direct current (DC), RF current does not directly stimulate nerves and therefore can be used without general anesthesia. RFA requires a complete electrical circuit to conduct current, and therefore if monopolar electrodes are used, grounding pads are required. RF current can pass through tissue with ionic fluid. Tissue is not a perfect conductor, and therefore RF current induces resistive heating in tissue, termed the Joule effect. Within several millimeters of the RF electrode, direct heating occurs, whereas more peripheral areas may be heated due to thermal conduction. For vein RFA, bipolar electrodes are used to allow current flow between electrodes with focused and effective heating. Grounding pads are not needed. 6
Laser, an acronym for light amplification by stimulated emission of radiation, involves the emission of single wavelength, monochromatic light with wavelengths ranging from short ultraviolet (<420 nm) to long far-infrared (10,600 nm). Laser light consists of waves that are in phase; rays are parallel and nondiverging, producing stronger biologic effect than ordinary light of the same power. 7 Like all radiation, upon interacting with a target tissue, laser energy is absorbed, scattered, or reflected. Absorption allows for potentially therapeutic or damaging tissue effects. The extent of absorption depends on the amount of chromophores in a tissue, such as hemoglobin, melanin, proteins, and water, which absorb light of specific wavelengths. 7 After absorbing photons, chromophores are excited to higher energy states; upon de-excitation, energy is released back into the tissue, resulting in photochemical and photothermolytic changes. Photothermolysis is the primary mechanism of tissue effect in EVLT. The extent of laser absorption and consequent energy release depends on the chromophores present, which may be targeted with laser light of specific wavelengths. 7
Scattering of laser light is also an important phenomenon determining its biologic effect. As it passes through matter, the light rays are scattered, reducing coherence and hence the effect of light on tissue. The extent of scattering determines the depth of laser light penetration, as tissue attenuates the incident beam. The amount of scattering is dependent on the nature of the chromophores and the wavelength of incident light. For example, light of longer wavelengths (1,470–1,500 nm) have much higher amounts of attenuation compared to light of shorter wavelengths (810–940 nm) in the vein wall, resulting in desirable greater tissue effect at the intended site of treatment. 7
6.3 Biological Effects of Thermal Ablation
The cellular response of tissue to heating is well described. Prolonged heating at 42 to 45°C causes reversible sublethal cellular damage. At temperatures exceeding 50 to 60°C, irreversible damage, including cellular necrosis, occurs. At temperatures approaching 100°C, water vaporizes, producing steam and desiccating tissue. Tissue charring and possibly melting occurs above 300°C. Typically, outer vein wall temperatures during EVLT are of the order of 32 to 100°C, and intraluminal temperatures near the probes are on average 729°C. 7 In RFA, the vein wall is heated to 85°C with RF energy, which is delivered by temperature feedback–controlled catheter electrodes. 8
Because blood, which normally fills the vein, and water, which is present in the vein wall and tissue surrounding the vein, cause a similar amount of attenuation of laser light, emptying the vein of blood is important for therapeutic effect, so that the biologic effect of the incident beam occurs on the vein wall itself rather than the blood within the vessel. This is accomplished during the procedure primarily by tumescent anesthesia, which constricts the vein by external compression and by inducing venospasm. 9
Multiple theories exist to account for the tissue effects of EVLT (see the following list). The steam bubble theory focuses on the hyperechoic bubbles seen with US at the fiber tip during energy application, and suggests that these represent boiling blood that has an indirect effect, heating the vein wall. The heat pipe theory maintains that blood in immediate contact with the fiber becomes coagulated, forming a clot around the tip that acts as an insulating layer, trapping and then conducting heat to the vein wall. The direct contact theory suggests that maximal heat transfer to the vein wall occurs when the laser fiber contacts the vein wall. This happens frequently over the course of treatment, as the vein is typically relatively tortuous. Segments between these sites receive energy by conduction. Likely, the effect of EVLT on the vein wall is due to a combination of these proposed mechanisms. 7
Proposed Mechanisms of Action for EVLT 10
Direct contact between the fiber tip and the vein wall.
Thermal interactions between the laser light and the vein wall:
Direct absorption of the scattered light by the vein wall.
Contact of heated blood with the vein wall.
Effect of steam bubbles produced by heated blood.
Effects of a carbonized blood layer attached to the fiber tip which absorbs light and becomes extremely hot damaging the vein wall.
Thermal injury of blood forming coagula within the vein lumen.
Histologic examination of veins removed immediately after thermal ablation demonstrates uneven vein wall destruction. The wall is perforated and ulcerated in areas of direct contact with the fiber tip, with relative sparing of the contralateral side. After 1 week, veins are thrombosed and demonstrate inflammatory tissue peripherally with migration of phagocytes and fibroblasts. There is uneven destruction of vein walls that is far more extensive than what is seen on veins harvested immediately after treatment. After 2 to 3 weeks, there is more organization of the perivenous inflammatory response, with scar tissue formation and proliferation of fibroblasts, myofibroblasts, and adventitial fibroblasts. 7
In contrast to EVLT, RFA results in more homogeneous, circumferential destruction of the vein wall. 11 In RFA, temperature feedback–controlled catheter electrodes sustain thermal energy in the vein wall, inducing collagen contraction and endothelia denudation. This results in contraction of the vein wall, in contrast to EVLT which induces vein closure predominantly by occlusion. 8 RFA does not induce damage as extensive and transmural as EVLT, probably because the temperature feedback mechanism of the generator promotes a more circular, homogeneous distribution of thermal energy, compared with the laser fiber tip. 11 In addition, RF has characteristic low-temperature treatments (90–120°C), as compared to the laser, which can cause boiling, vaporizing, and carbonization of tissues (700–1,500°C). 12
6.4 Endovenous Laser Therapy
EVLT can be performed as an outpatient treatment using tumescent anesthesia and under US guidance. No preprocedural laboratory work-up is required. Lidocaine allergy is a relative contraindication to the procedure.
6.4.1 Equipment
Tumescent anesthesia can be delivered manually using a spinal needle, or with the use of a HK Klein Infiltration pump (HK Surgical Inc). The standard concentration of tumescent consists of normal saline and 1% lidocaine for a solution of 0.1% lidocaine mixed with normal saline. When performing bilateral EVTA, one must be cognizant of lidocaine toxicity and reduce the lidocaine concentration in the solution to 0.05%, which has been demonstrated to be safe in the outpatient setting. 13
Several EVLT devices are available for clinical use, which are approved by Food and Drug Administration ( ▶ Table 6.1). Bare laser tip design has been traditionally used for different wavelengths, but because there is direct contact of the laser tip with the vein wall, associated vessel perforation occurs resulting in pain and bruising. In order to prevent that, other designs had been implemented since 2010, such as gold-tip, radial, and tulip-tip fibers. For example, covered or jacket-tip fibers eliminate the contact of the laser tip with the vessel wall, decreasing the risk of wall perforation and minimizing discomfort and bruising ( ▶ Fig. 6.1). US guidance is performed with grayscale and Doppler evaluation with linear transducers.

Fig. 6.1 Example of jacket-tip fiber (Angiodynamics NeverTouch Gold-Tip fiber).
Laser λ | Device | Manufacturer |
810 nm | Ceralas D15/810 | Biolitec, East Longmeadow, MA |
Vari-Lase | Vascular Solutions, Minneapolis, MN | |
980 nm | Ceralas D, EVOLVE | Biolitec, East Longmeadow, MA |
Precision 980 | Angiodynamics, Queensbury, NY | |
1,320 nm | CoolTouch CTEV | Cooltouch Corp., Roseville, CA |
1,470 nm | VenaCure | Angiodynamics, Queensbury, NY |
ELVeS | Biolitec, East Longmeadow, MA |
6.4.2 Treatment Protocols with Different Wavelengths
Endovenous laser obliteration employs one of several different wavelengths (810, 940, 980, 1,320, 1,470, and 1,500 nm) to produce thermal energy. 14 The thermal efficacy of EVLT is driven by the laser power, and different wavelengths deliver this energy to the vein wall differently: theoretically, the energy delivered by 1,470- to 1,500-nm wavelength light to the vein wall is much higher compared with 810- to 980-nm lasers, leading to greater selective destruction. This means that less power is needed by higher wavelengths in comparison with lower wavelengths to achieve the same thermal effect in the vein wall. Linear endovenous energy density (LEED) is the ratio of laser power to the velocity of the laser fiber pullback performed during the procedure and is measured in joules per centimeter (J/cm). Accordingly, lasers with higher wavelengths need a lower LEED (30–70 J/cm) to achieve the same results of lasers with lower wavelengths (70–120 J/cm). 7 When the laser power and the pullback velocity of the laser fiber are reported, the resulting LEED in J/cm serves as a parameter for reproducibility of the procedure, as other phlebologists can use the same protocol. In addition to wavelength, vein size also determines the amount of energy used because some authors may increase the LEED when treating veins with larger diameters. 10, 15
Longer wavelength devices of 1,470 to 1,500 nm have been proposed more recently because these wavelengths are preferentially absorbed by water, which would theoretically target the vein wall instead of blood. However, there is controversy regarding the power needed for treatment, and more research is needed to evaluate the efficacy and safety of these devices. 9 All current available laser devices have proven to be successful in closure of veins with high success rates (>90%), independently of the wavelengths. There are few studies comparing different energy per centimeter of vein treated and protocols required for durable vein occlusion, with a suggested minimum threshold of 38 J/cm for veins < 6 mm in diameter and 63 J/cm in veins with a 10-mm diameter. 16
6.4.3 Endovenous Laser Ablation Technique
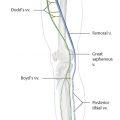
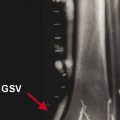
Before the ablation, duplex US is performed to map incompetent sources of venous reflux, determining the incompetent portions of the great saphenous vein (GSV) starting at the SFJ, or the incompetent small saphenous vein (SSV) relative to the saphenopopliteal junction (SPJ).
Informed consent should be obtained, including a discussion of risks and benefits and other treatment alternatives. Although absolute contraindications for this procedure remain to be determined, relative contraindications for the EVTA as defined by the Society of Interventional Radiology guidelines include the following: pregnancy or nursing, obstructed deep venous system inadequate to support venous return after EVTA, liver dysfunction, allergy to lidocaine, severe uncorrectable coagulopathy, inability to wear compression stockings, inability to ambulate after the procedure, sciatic vein reflux, and nerve stimulator. 17
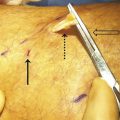
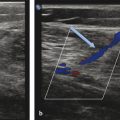
The physician performing the procedure should wear gown and eye protection (including laser-specific attenuating glasses for the operator, patient, and staff; ▶ Fig. 6.2), and all staff in the procedural room should follow universal precautions. After consent is obtained, the patient is placed supine (for GSV treatment) or prone (for SSV treatment), and the region to be treated is sterilely prepared and isolated with sterile barriers ( ▶ Fig. 6.3). To maximize visualization of varicose veins, patients are placed in reverse Trendelenburg’s position just before US-guided puncture.

Fig. 6.2 Example of different protection glasses. The use of protective eyewear is required for every person in the procedure room while the laser is activated, including the operator, support staff, and patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree