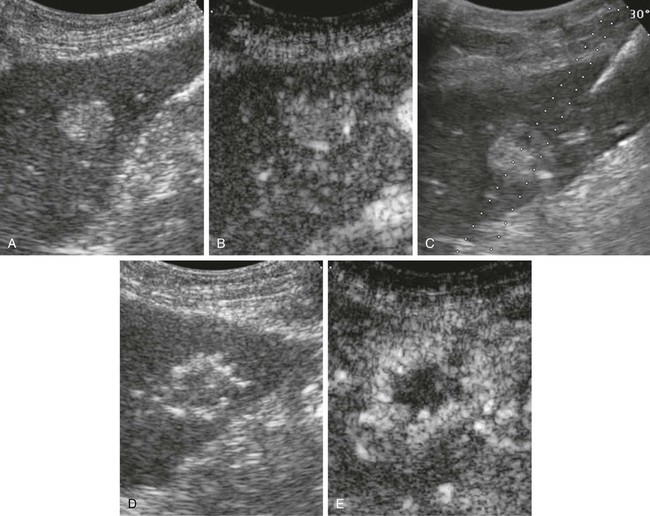
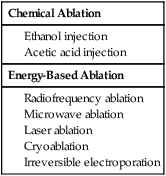
Riccardo Lencioni, Elena Bozzi, Laura Crocetti and Carlo Bartolozzi Hepatocellular carcinoma (HCC) is the fifth most common cause of cancer, and its incidence is increasing worldwide because of the dissemination of hepatitis B and C virus infection.1,2 Patients with cirrhosis are at the highest risk of developing HCC and should be monitored every 6 months to diagnose the tumor at an asymptomatic stage.2 If diagnosed at an early stage, patients should be considered for any of the available options that may provide a high rate of complete response. These include surgical resection, liver transplantation, and percutaneous techniques of tumor ablation.3 Indication for surgical resection is currently restricted to patients with single asymptomatic HCC and extremely well-preserved liver function who have neither clinically significant portal hypertension nor abnormal bilirubin.4,5 Cadaveric liver transplantation is limited by the shortage of donors, and living donor liver transplantation is still at an early stage of clinical application.4,5 As a result, percutaneous ablation plays a key role in therapeutic management of HCC. In the setting of a patient with known hepatitis B or cirrhosis of other etiology, a solid nodular lesion found during surveillance has a high likelihood of being HCC. However, it has been shown by pathologic studies that many small nodules detected in cirrhotic livers do not correspond to HCC.6 Current guidelines recommend further investigation of nodules detected during surveillance with dynamic imaging techniques, including contrast-enhanced multidetector computed tomography (MDCT) and contrast-enhanced magnetic resonance imaging (MRI).4 In fact, one of the key pathologic factors for differential diagnosis that is reflected in dynamic imaging studies is the vascular supply to the lesion. Through the progression from regenerative nodule, to low-grade dysplastic nodule (DN), to high-grade DN, to frank HCC, one sees loss of visualization of portal tracts and development of new arterial vessels, termed nontriadal arteries, which become the dominant blood supply in overt HCC lesions.6 It is this neovascularity that allows HCC to be diagnosed and is the key for imaging cirrhotic patients. A rational diagnostic protocol should be structured according to the actual risk of malignancy and the possibility of achieving a reliable diagnosis. Since the prevalence of HCC among ultrasound-detected nodules is strongly related to the size of the lesion, the diagnostic workup depends on lesion size. Lesions smaller than 1 cm in diameter have a low likelihood of being HCC, but minute hepatic nodules detected by ultrasound may become malignant over time. Therefore, these nodules should be followed up to detect growth suggestive of malignant transformation. A reasonable protocol is to repeat ultrasound every 3 months until the lesion grows to more than 1 cm, at which point additional diagnostic techniques are applied.4 It has to be emphasized, however, that the absence of growth during the follow-up period does not rule out the malignant nature of the nodule, because even an early HCC may take more than 1 year to increase in size.4 When the nodule exceeds 1 cm in size, the lesion is more likely to be HCC, and diagnostic confirmation should be pursued. Current guidelines recommend that the diagnosis of HCC can be made noninvasively without biopsy in a nodule larger than 1 cm that shows the characteristic vascular features of HCC (i.e., arterial hypervascularization with washout in the portal venous or delayed phase) even in patients with normal α-fetoprotein values. Such lesions should be treated as HCC, since the positive predictive value of the clinical and radiologic findings exceeds 95%, provided that examinations are conducted by using state-of-the-art equipment and interpreted by radiologists with extensive expertise in liver imaging.7 For lesions above 1 cm without a characteristic vascular profile, a second dynamic study is required. If definitive diagnosis is not reached with these techniques, biopsy is recommended.4 It has to be pointed out that noninvasive criteria based on imaging findings can be applied only in patients with established cirrhosis.4 For nodules detected in noncirrhotic livers, as well as for those showing atypical vascular patterns, biopsy is recommended. Ultrasound is widely accepted for HCC surveillance, but multidetector CT or dynamic MRI are required for intrahepatic staging of the disease; these examinations provide a comprehensive assessment of the liver parenchyma and can identify additional tumor foci. In most solid malignancies, tumor stage at presentation determines prognosis and treatment management. Most patients with HCC, however, have two diseases—liver cirrhosis and HCC—and complex interactions between the two have major implications for prognosis and treatment choice.8 Therefore, the TNM system has limited usefulness in the clinical decision-making process because it does not take into account hepatic functional status. Several scoring systems have been developed in the past few years in attempts to stratify patients according to expected survival. However, the only system that links staging with treatment modalities is the Barcelona Clinic Liver Cancer (BCLC) staging system.9,10 The BCLC includes variables related to tumor stage, liver functional status, physical status, and cancer-related symptoms and provides an estimation of life expectancy that is based on published response rates to the various treatments. In the BCLC system, early-stage HCC includes patients with World Health Organization (WHO) performance status of 0, preserved liver function (Child-Pugh class A or B), and solitary tumor or up to 3 nodules smaller than 3 cm each, in the absence of macroscopic vascular invasion and extrahepatic spread. If the patient has Child-Pugh class A cirrhosis and a solitary tumor smaller than 2 cm, the stage may be defined as very early. Patients with multinodular HCC with neither vascular invasion nor extrahepatic spread are classified as intermediate-stage according to the BCLC staging system, provided they have a performance status of 0 and Child-Pugh class A or B cirrhosis.11 Patients with portal vein invasion or extrahepatic disease are classified as advanced stage. The terminal stage includes patients who have either severe hepatic decompensation (Child-Pugh class C) or performance status greater than 2. Patients with early-stage HCC can benefit from curative therapies including surgical resection, liver transplantation, and percutaneous ablation, and have the possibility of long-term cure, with 5-year survival figures ranging from 50% to 75%.10 However, there is no firm evidence to establish the optimal first-line treatment for early-stage HCC because of the lack of randomized controlled trials (RCTs) comparing radical therapies. Patients should be evaluated in referral centers by multidisciplinary teams involving hepatologists, oncologists, interventional radiologists, surgeons, and pathologists to guarantee careful selection of candidates for each treatment option and ensure expert application of these treatments.10 Resection is the treatment of choice for HCC in noncirrhotic patients, who account for about 5% of the cases in Western countries. However, in patients with cirrhosis, candidates for resection have to be carefully selected to reduce the risk of postoperative liver failure. It has been shown that a normal bilirubin concentration and the absence of clinically significant portal hypertension are the best predictors of excellent outcomes after surgery.12 In experienced hands, such patients have treatment-related mortality of less than 1% to 3% and may achieve 5-year survival higher than 70%.12–14 In contrast, survival drops to less than 50% at 5 years in patients with significant portal hypertension, and to less than 30% at 5 years in those with both adverse factors (portal hypertension and elevated bilirubin).12 Most groups restrict the indication for resection to patients with a single tumor in a suitable location. Anatomic resections guided by intraoperative ultrasound techniques are preferred to wedge resections, because they include any microsatellite lesions possibly located in the same hepatic segment as the main tumor. In fact, it is known that neoplastic dissemination occurs at very early stages in HCC via the invasion of small peripheral portal vein branches.6 After resection, tumor recurrence rate exceeds 70% at 5 years, including recurrence due to dissemination and de novo tumors developing in the remnant cirrhotic liver. The most powerful predictors of recurrence are the presence of microvascular invasion and/or additional tumor sites besides the primary lesion.12 Liver transplantation is the only option that provides cure of both the tumor and the underlying chronic liver disease. It is recognized as the best treatment for patients with solitary HCC smaller than 5 cm in the setting of decompensated cirrhosis and for those with early multifocal disease (up to 3 lesions, none larger than 3 cm).4 However, for patients with a solitary small tumor in well-compensated cirrhosis, the optimal treatment strategy is still under debate.15 The reported outcomes of patients who actually underwent transplantation are better than those of patients submitted to resection, especially if the substantially lower rates of tumor recurrence (<10%-20% at 5 years) are considered.15 Overall survival, however, decreases from an intention-to-treat perspective.12,15–17 In fact, because of the lack of sufficient liver donation, there is always a waiting period between listing and transplantation during which the tumor may grow and develop contraindications to transplantation (vascular invasion, extrahepatic spread). The rate of dropouts may be as high as 25% if the waiting list is longer than 12 months.18 Most groups perform interventional treatments, including transarterial chemoembolization (TACE) and percutaneous ablation, to achieve local control of the tumor during the waiting period. Living donor liver transplantation is a viable option to expand the number of available livers, but it requires a highly skilled group of senior liver surgeons, increases surgery-related morbidity, and carries the risk of donor mortality. In addition, the applicability of the technique is low, and only about one fourth of potential recipients eventually undergo the procedure.15 Image-guided percutaneous ablation is currently accepted as the best therapeutic choice for nonsurgical patients with early-stage HCC.4 Over the past 2 decades, several methods for percutaneous chemical ablation or energy-based tumor destruction through localized heating, freezing, or energy delivery have been developed and clinically tested (Table 141-1). TABLE 141-1 Percutaneous Methods for Ablation of Hepatocellular Carcinoma The seminal technique used for local ablation of HCC is percutaneous ethanol injection (PEI). Ethanol induces coagulation necrosis of the lesion as a result of cellular dehydration, protein denaturation, and chemical occlusion of small tumor vessels. PEI is a well-established technique for the treatment of nodular-type HCC. HCC nodules have a soft consistency and are surrounded by a firm cirrhotic liver. Consequently, injected ethanol diffuses within them easily and selectively, leading to complete necrosis of about 70% of small lesions.19 Although there have been no RCTs comparing PEI and best supportive care or PEI and surgical resection, several retrospective studies have provided indirect evidence that PEI improves the natural history of HCC. The long-term outcomes of patients with small tumors who were treated with PEI were similar to those reported in surgical series, with 5-year survival rates ranging from 41% to 60% in Child A patients.19–24 Of importance, two cohort studies and one retrospective case-control study comparing surgical resection and PEI failed to identify any difference in survival, despite patients in PEI groups having poorer liver function.25–27 The major limitation of PEI is the high local recurrence rate, which may reach 33% in lesions smaller than 3 cm and 43% in lesions exceeding 3 cm.28,29 The injected ethanol does not always accomplish complete tumor necrosis because of its inhomogeneous distribution within the lesion—especially in presence of intratumoral septa—and the limited effect on extracapsular cancerous spread. Moreover, PEI is unable to create a safety margin of ablation in the liver parenchyma surrounding the nodule, and therefore may not destroy tiny satellite lesions that even in small tumors may be located in close proximity to the main nodule. The thermal damage caused by heating is dependent on both the tissue temperature achieved and the duration of heating. Heating of tissue at 50°C to 55°C for 4 to 6 minutes produces irreversible cellular damage. At temperatures between 60°C and 100°C, near-immediate coagulation of tissue is induced, with irreversible damage to mitochondrial and cytosolic enzymes of the cells. At more than 100°C to 110°C, tissue vaporizes and carbonizes.30 For adequate destruction of tumor tissue, the entire target volume must be subjected to cytotoxic temperatures. Different physical mechanisms are involved in the hepatic hyperthermic treatments to generate a lethal temperature. A common important factor that affects the success of thermal ablation is the ability to ablate all viable tumor tissue and possibly an adequate tumor-free margin. Ideally, a 360-degree, 0.5- to 1-cm-thick ablative margin should be produced around the tumor.30 This cuff would ensure that microscopic invasions around the periphery of a tumor have been eradicated. Thus, the target diameter of an ablation, or of overlapping ablations, must be larger than the diameter of the tumor that undergoes treatment.31 Thermal ablation is usually performed under intravenous sedation with standard cardiac, pressure, and oxygen monitoring. Targeting of the lesion can be performed with ultrasound, CT, or MR imaging. The guidance system is chosen largely on the basis of operator preference and local availability of dedicated equipment such as CT fluoroscopy or open MR systems. Real-time ultrasound/CT (or ultrasound/MRI) fusion imaging systems recently developed can substantially improve the ability to guide and monitor liver tumor ablation procedures. Current virtual navigation systems help define the extent of liver tumor burden, plan and simulate needle insertion, and predict the amount of induced necrosis. During the procedure, important aspects to be monitored include how well the tumor is being covered and whether any adjacent normal structures are being affected at the same time. Although the transient hyperechoic zone seen at ultrasound within and surrounding a tumor during and immediately after RFA can be used as a rough guide to the extent of tumor destruction, MR is currently the only imaging modality with validated techniques for real-time temperature monitoring. To control an image-guided ablation procedure, the operator can use the image-based information obtained during monitoring or automated systems that terminates the ablation at a critical point in the procedure. At the end of the procedure, most systems allow ablation of the needle track, which is aimed at preventing any tumor cell dissemination. Contrast-enhanced ultrasound performed after the end of the procedure may allow an initial evaluation of treatment effects (Fig. 141-1). However, CT or MRI are recognized as the standard modalities to assess treatment outcome, showing, in cases of successful ablation, a nonenhancing area with or without a peripheral enhancing rim. The enhancing rim that may be observed along the periphery of the ablation zone appears as a relatively concentric, symmetric, and uniform process in an area with smooth inner margins. This is a transient finding that represents a benign physiologic response to thermal injury (initially, reactive hyperemia; subsequently, fibrosis and giant cell reaction). Benign periablational enhancement has to be differentiated from irregular peripheral enhancement due to residual tumor that occurs at the treatment margin. In contrast to benign periablational enhancement, residual unablated tumor often grows in scattered, nodular, or eccentric patterns.32 Later follow-up imaging studies should be aimed at detecting recurrence of the treated lesion (i.e., local tumor progression), development of new hepatic lesions, or emergence of extrahepatic disease. Several electrode types are available for clinical RFA, including internally cooled electrodes and multi-tined expandable electrodes with or without perfusion.32 Cooled-tip electrodes consist of dual-lumen needles with uninsulated active tips in which internal cooling is obtained by continuous perfusion with chilled saline. Needle cooling is aimed at preventing overheating of tissues nearest to the electrode, which may cause charring, thereby limiting the propagation of RF waves. They are available in either single-needle or cluster array with three needles spaced 0.5 cm apart. Expandable needles have an active surface that can be substantially expanded by hooks deployed laterally from the tip. The number of hooks and length of hook deployment may vary according to the desired volume of necrosis. These techniques enabled a substantial and reproducible enlargement of the volume of thermal necrosis produced with a single-needle insertion and prompted the start of clinical application of RFA.
Energy-Based Ablation of Hepatocellular Cancer
Pretreatment Evaluation
Imaging Assessment
Clinical Staging
Treatment of Early-Stage Hepatocellular Carcinoma
Surgical Resection
Liver Transplantation
Image-Guided Ablation
Chemical Ablation
Energy-Based Ablation
Technical Aspects
Thermal Ablation
Radiofrequency Ablation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Energy-Based Ablation of Hepatocellular Cancer