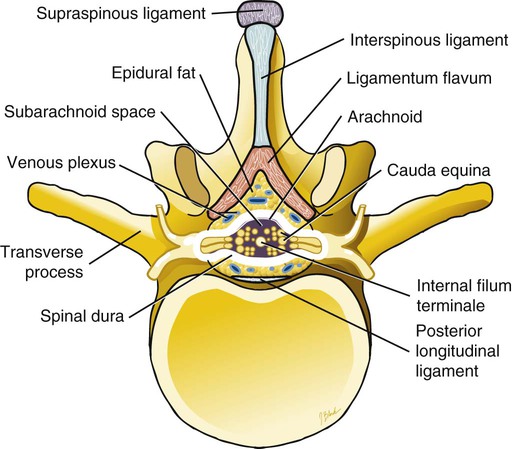
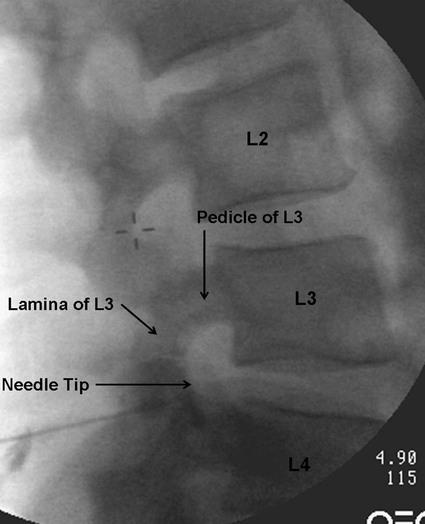
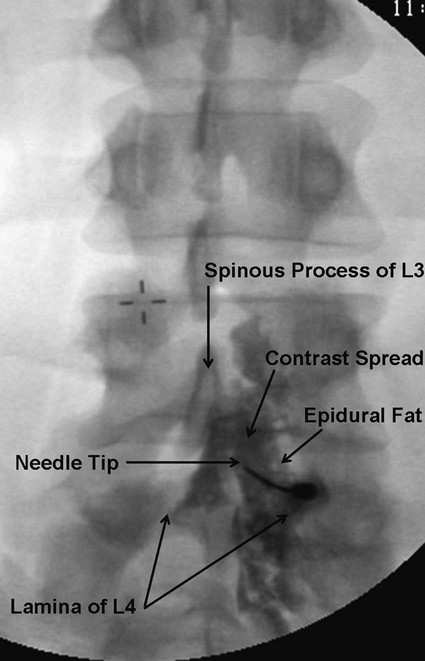
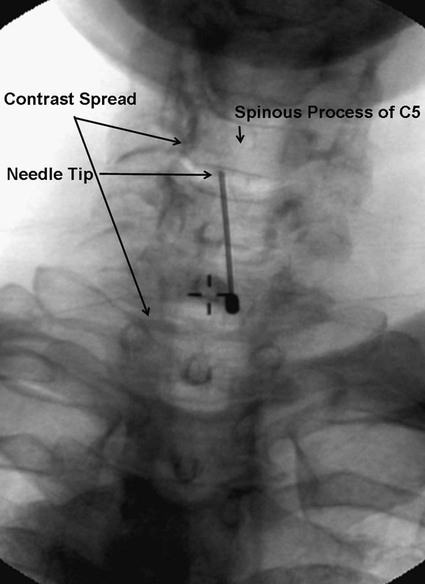
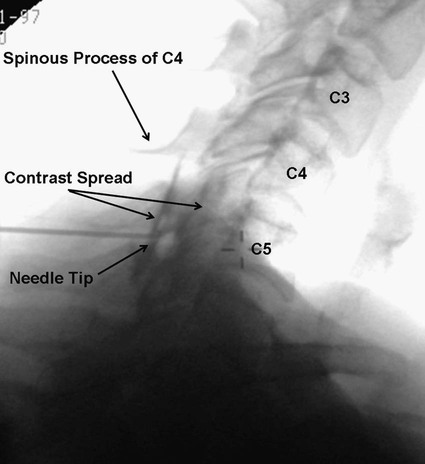
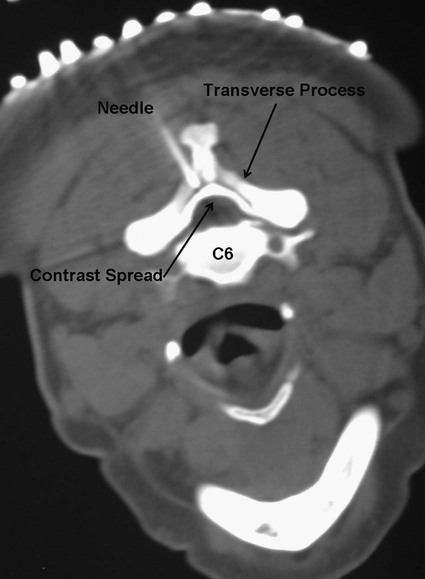
Low back pain is a significant medical problem. It is widely prevalent, affecting patients of all ages, and greatly contributes to healthcare and disability costs. Bressler et al. estimated the frequency of back pain at 13% to 49% of patients over the age of 65.1 Overall prevalence is 60% to 80%, moreso in industrialized countries, with peak prevalence between 45 and 60 years of age.1–3 Low back pain disorders are expensive, accounting for one third of all disability costs in the United States. Of patients on disability, low back pain represents only 3% of subjects receiving compensation, but they receive 75% of payments. In 2006, the economic burden of back and neck pain on the United States was estimated at $85.9 billion and was increasing faster than the overall rate of medical inflation.4 Patients with back pain had nearly twice the medical expenditures as patients without back pain.4 Lumbosacral radiculopathy, commonly known as sciatica, presents with a wide range of symptoms from mild intermittent low back and leg pain to severe neurologic compromise. Symptoms arise from mechanical compression of the lumbosacral nerve roots. Younger patients are more likely to suffer from intervertebral disc herniation, whereas older patients may have vertebral degeneration, facet joint hypertrophy, spondylosis, and osteophytes as the cause of their neuroforaminal stenosis, radiculopathy, and/or pain. Conservative treatment includes rest, oral antiinflammatory medications, oral corticosteroids, analgesics, and physical therapy.5,6 The natural course of radicular pain is variable but typically resolves in a matter of weeks to months.7 During this time, epidural steroid injection (ESI) can be helpful in alleviating symptoms and promoting a return to normal function. In complicated cases, surgery may be necessary to relieve nerve root compression. ESI can be an effective alternative to surgery in some patients and provide relief in cases where surgery has failed.8 • Radiculopathy/radiculitis: from herniated nucleus pulposus, degenerative stenosis • Spinal stenosis: central or neuroforaminal Radiculopathy is the best indication for ESI and has the best outcomes. A careful history and physical can optimize patient selection for ESI. Back pain or neck pain with radiation to the extremity, a dermatomal pattern of sensory loss, and presence of sciatic or brachial plexus stretch signs are symptoms of radiculitis and may predict better success with ESI.9 In contrast, non-radiating axial pain, myofascial pain syndrome, facet and sacroiliac arthropathy, and neurogenic claudication respond less well to ESI.10 However, there is a paucity of effective treatments for these pain syndromes and a huge prevalence of them, so ESI is often done as a trial of therapy. Other axial pain treatments, such as sacroiliac joint steroid injections, zygapophyseal (facet) joint injection, and median branch denervation, can be effective if pain persists despite ESI. Although there is a poor correlation between outcomes and imaging studies, computed tomography (CT) and magnetic resonance imaging (MRI) can be important tools for localizing the area of nerve root compression and ruling out other serious causes of back pain such as tumor.2,9 • Coagulopathy, thrombocytopenia, or anticoagulant therapy • Relative contraindications are uncontrolled diabetes, glaucoma, and immunocompromise. Spinal/epidural hematoma and abscess are the major complications of epidural injection to be avoided. ESIs are never life saving, so any patient with overt risk for bleeding or infection should be postponed. Immunocompromised patients, such as those with HIV or cancer, may be at higher risk for infection, so extra care should be taken both in patient selection and technique for them. Injected steroids can worsen glaucoma and glucose control in diabetics.9,11 • Touhy needle for midline injection, 17 to 18 gauge for lumbar, 20 gauge for cervical injection • Spinal needle (22 gauge) for transforaminal or caudal injection • Extension tubing for transforaminal injection • Injectable contrast solution such as Omnipaque or Isovue • Steroid preparation (triamcinolone diacetate 40-80 mg, methylprednisolone acetate 40-80 mg, betamethasone 6-12 mg, or dexamethasone 8-16 mg) • Preservative-free local anesthetic (lidocaine or bupivacaine) or saline for steroid diluent • Intravenous access for cervical or thoracic injections • Resuscitation and airway equipment • Monitors, including pulse oximetry, blood pressure, and electrocardiography An understanding of the anatomy of the spinal column including the vertebrae, spinal cord and nerve roots, and ligamentous structures is necessary. The lumbar vertebra is comprised of a spinous process, two laminae, two transverse processes, two pedicles, and a vertebral body. Circumferentially, these structures make up the spinal canal, which contains the dural sac, spinal cord, cauda equina, and cerebrospinal fluid (CSF). Ligaments, from posterior to anterior, are the supraspinous, interspinous, and ligamentum flavum. The epidural space lies deep to the ligamentum flavum and is a potential space containing fat, blood vessels, nerve roots, and dural sac (Fig. 168-1). The epidural space extends laterally to the intervertebral foramen and anteriorly to the vertebral body. The inferior border of the epidural space is at the sacral hiatus, a U-shaped space between the inferior aspect of the sacrum and the coccyx. The hiatus is bounded laterally by the sacral cornua, with the sacrococcygeal ligament as the main structure between the skin and epidural space at this level.12 For the safe performance of transforaminal injections, knowledge of neuroforaminal anatomy is critical. The neuroforamen is bordered posteriorly by the zygapophyseal joints, superiorly and inferiorly by the pedicles, and anteriorly by the vertebral bodies and intervertebral disk. The neuroforamen contains the exiting nerve root in a dural sleeve and the radicular artery. At some levels, the radicular artery becomes the main blood supply to the anterior spinal cord and is then termed the major anterior radicular artery (artery of Adamkiewicz). The artery of Adamkiewicz usually arises from one of the left intercostal arteries, typically between T8 and L3,13,14 but many different anatomic variations have been reported, including an origination from the lower lumbar segments.15 The nerve root is thought to lie at the superior anterior aspect of the neuroforamen most (but not all) of the time, and the radicular artery is attached to the ventral surface of the nerve root. Previously, the superior and posterior aspect of the neuroforamen had been promoted as the “safe triangle” for transforaminal injection. However, a recent study of 113 subjects showed that the artery of Adamkiewicz lies in the superior half of the foramen 97% of the time. Thus placing the needle in the inferior half should reliably miss the artery.14 The intervertebral disk also lies in the inferior half of the foramen, so if this site is chosen for needle placement, care should be taken not to advance the needle too deeply and inject the disc annulus or nucleus. Intradiscal injection could be less effective, since the medications would not be applied to the inflamed nerve and may carry higher risks due to the potential for discitis or incitement of degenerative disk disease. The patient is positioned prone with a pillow under the lower abdomen to straighten the curve of the lumbar spine and open the interlaminar space. An anteroposterior (AP) fluoroscopic view is used to identify the targeted level in the midline. After sterile preparation and draping, the skin is infiltrated with lidocaine. A 17-, 18-, or 20-gauge Touhy needle with stylet is carefully inserted through the anesthetized area of skin and advanced to the supraspinous ligament. At this point, there will be greater resistance to needle advancement as the needle engages the dense fibers of the ligament. Fluoroscopy should be used to verify midline positioning of the needle. The stylet is removed, and a well-lubricated 5-mL syringe containing preservative-free saline or air is attached. A loss of resistance technique is used along with radiologic guidance. The needle is advanced slowly and incrementally through the ligaments. As the needle tip reaches the ligamentum flavum, even greater resistance will be encountered. Once the needle traverses the ligamentum flavum, there will be a loss of resistance to both needle advancement and syringe injection. Lateral fluoroscopy or CT guidance is very helpful in identifying the true epidural space (Fig. 168-2). Once in the epidural space, the needle tip will be just anterior to the laminae and spinous processes. If aspiration is negative for blood and CSF, 0.5 to 3 mL of contrast is injected. Contrast solution should spread along the posterior epidural space and should be confirmed in the AP view (Fig. 168-3). Dural puncture, or “wet tap,” is usually evident by the free flow of CSF and layering of contrast anteriorly along the vertebral body. Occasionally, dural puncture may occur unnoticed, or subdural extraarachnoid injection can occur.16,17 In the first case, patients may report postdural puncture headache later (see later) or have immediate temporary weakness from spinal block from any injected local anesthetic. Subdural injection presents as a slow onset (10-15 minutes) of spinal block with weakness, sensory loss, and hypotension.17 ESI is also effective in the cervical spine, but with an increased risk of both minor and severe complications. In the cervical spine, meticulous care should be taken to avoid dural puncture. Cervical dural puncture can lead to persistent CSF leak and severe persistent headache.18 Beyond dural puncture, direct and catastrophic spinal cord injury can also result from anterior needle positioning. Radiologic guidance should always be used for cervical injection. If the needle tip appears too anterior, without loss of resistance, it may be lateral and should be repositioned. Also, the ligamentum flavum is much thinner in the cervical spine, so the tactile response during needle advancement will be less. In some cases, the ligamentum flavum fails to fuse and is completely absent.19 Injecting a small amount (0.3 mL) of contrast can reveal whether the needle is in the epidural space or still too posterior (Figs. 168-4 through 168-6). Once epidural positioning is verified, the steroid mixture is slowly injected. There should be virtually no resistance to injection; any change in resistance warrants repeating the procedure at a different level. Patients often report pressure or mild discomfort during injection. However, complaints of pain should prompt the physician to stop injection and consider moving to a different level or using a smaller volume of injectate. Steroids should be diluted with preservative-free saline. Using local anesthetic as a diluent carries a risk of unrecognized subdural injection and late onset of cervical spinal or epidural block. In that event, the diaphragm and respiratory function could be compromised. In the cervical spine, total volume of injectate should be 3 to 6 mL, and in the lumbar spine, volume can be as little as 5 mL for an elderly patient with severe spinal stenosis or as much as 15 mL for a young patient with a herniated intervertebral disk. Thoracic epidural steroid injection can also be done but is rarely needed.20
Epidural Steroid Injection
Clinical Relevance
Indications
Contraindications
Equipment
Technique
Anatomy
Approach
Interlaminar
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Epidural Steroid Injection