Fig. 10.1
Barrett’s esophagus (endoscopic view) (a) native (b) after staining with methylene blue
10.5 Classification and Pathology
The TNM staging system (7th edition) as outlined by Union Internationale Contre le Cancer (UICC) and corresponding American Joint Committee on Cancer (AJCC) stage groups esophageal cancer in different stages (Table 10.1) [10]. AC of the EGJ is classified best according to Siewert et al. [11]. Type I tumors (AC of the distal esophagus), type II tumors (true carcinoma of the cardia) and type III tumors (subcardial gastric cancer infiltrating the distal esophagus) can be distinguished (Fig. 10.2). Histologically, epithelial carcinomas of the esophagus can be subgrouped into squamous cell carcinoma (ICD 0-M 8070/3), verrucous (squamous) carcinoma (ICD 0-M 8051/3), basaloid squamous cell carcinoma (ICD 0-M 8083/3), spindle cell (squamous) carcinoma (ICD 0-M 8074/3), adenocarcinoma (ICD 0-M 8140/3), adenosquamous carcinoma (ICD 0-M 8560/3), mucoepidermoid carcinoma (ICD 0-M 8430/3), adenoid cystic carcinoma (ICD 0-M 8200/3), small cell carcinoma (ICD 0-M 8041/3) and undifferentiated carcinoma (ICD 0-M 8020/3) [12].
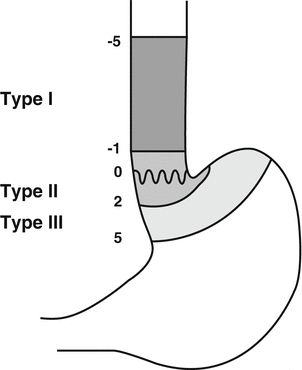
Table 10.1
TNM- and UICC-classification of esophageal cancer 2010
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
Tis | Carcinoma in situ/High grade dysplasia | ||
T1a | Tumor invades lamina propria or muscularis mucosae | ||
T1b | Tumor invades submucosa | ||
T2 | Tumor invades muscularis propria | ||
T3 | Tumor invades adventitia | ||
T4a | Tumor invades pleura, pericardium, diaphragm or adjacent peritoneum | ||
T4b | Tumor invades neighbouring structures, such as aorta, vertebral body or Trachea | ||
NX | Regional lymph nodes cannot be assessed | ||
N0 | No regional lymph node metastasis | ||
N1 | 1–2 regional lymph node metastases | ||
N2 | 3–6 regional lymph node metastases | ||
N3 | ≥7 regional lymph node metastases | ||
MX | Distant metastasis cannot be assessed | ||
M0 | No distant metastasis | ||
M1 | Distant metastasis | ||
Stage 0 | Tis | N0 | M0 |
Stage IA | T1 | N0 | M0 |
Stage IB | T2 | N0 | M0 |
Stage IIA | T3 | N0 | M0 |
Stage IIB | T1,T2 | N1 | M0 |
Stage IIIA | T4a | N0 | M0 |
T3 | N1 | M0 | |
T1,T2 | N2 | M0 | |
Stage IIIB | T3 | N2 | M0 |
Stage IIIC | T4a | N1, N2 | M0 |
T4b | Any N | M0 | |
Any T | N3 | M0 | |
Stage IV | Any T | Any N | M1 |

Fig. 10.2
Siewert classification of GEJ tumors
The most common sites of metastases of esophageal SCC are the regional lymph nodes. The risk for lymph node metastases is about 5 % if the tumor is confined to the mucosa, 30 % if the tumor invades submucosa and over 80 % if the tumor invades adjacent organs/tissues. Lesions of the upper third of the esophagus most frequently involve cervical and mediastinal lymph nodes, whereas those of the middle third metastasize to the mediastinal, cervical and upper gastric lymph nodes. Carcinomas of the lower third preferentially spread to the lower mediastinal and the abdominal lymph nodes. The most common sites of hematogenous metastases are the lung and the liver. Less frequently affected sites are the bones, adrenal glands, and brain [12]. AC spread first locally and infiltrate the esophageal wall. Distal spread to the stomach may occur. Extension through the esophageal wall into adventitial tissue, and then into adjacent organs or tissues is similar to SCC. Common sites of local spread comprise the mediastinum, tracheobronchial tree, lung, aorta, pericardium, heart and spine. Barrett associated AC metastasize to para-esophageal and paracardial lymph nodes, those of the lesser curve of the stomach and the celiac nodes. Distant metastases occur rather late [12].
10.6 Molecular Mechanisms
Many genes such as EGFR (epithelial derived growth factor receptor), cyclin D1, the tumor suppressors p16, and p53 (mutated in 35–80 % in SCC) have been found to play a role in the development of SCC and AC but the underlying exact mechanisms by which this disease develops are still not clear [13, 14]. Recently, a team of researchers at the University of Texas MD Anderson Cancer Center reported that the mTOR molecular pathway promotes the activity of the Gli1 transcription factor – a protein that moves into the cell nucleus where it binds to and activates other genes. Gli1 normally is held out of the nucleus by a protein called SuFu, which binds to it at a specific region. The Hedgehog pathway frees Gli1 by activating a signaling protein called Smoothened (SMO), which blocks SuFu binding, allowing Gli1 to move into the nucleus and activate a variety of genes, including Hedgehog activators. Thus, the research group established a cross-talk between both pathways promoting esophageal cancer development and progression [15]. An analysis of 107 tissue samples of human esophageal cancer tumors showed that 80 (74.8 %) had a marker of mTOR promotion of Gli1 and 87 (81.3 %) had the version of Gli1 activated by Hedgehog. Earlier research by other labs indicates that the AKT and MAPK/ERK pathway also activate the Hedgehog pathway. Wang and colleagues [15] showed that AKT and ERK, which both activate the mTOR pathway, appear to activate Gli1 via phosphorylation of S6K1 and Gli1.
10.7 Clinical Symptoms
The major symptom of esophageal cancer is dysphagia, less common odynophagia. Most of the patients experience dysphagia at a late stage of disease. Hematemesis, hoarseness as a result of paresis of the recurrent laryngeal nerve, respiratorial symptoms caused by tracheoesophageal fistula, loss of weight and swollen cervical lymph nodes are late symptoms. Heartburn it a typical symptom of patients with Barrett carcinoma. However, surprisingly many patients with Barrett carcinoma do not report on a long history of heartburn. One may speculate that intestinal Barrett metaplasia tolerates acid reflux much better. Thus, the patients do not suffer from heartburn.
10.8 Diagnostic Tools
The diagnosis should be made from an endoscopic biopsy with the histology to be given according to World Health Organization (WHO) criteria. Staging should include clinical examination, blood counts, liver-, pulmonary- and renal function tests, endoscopy (including upper-aerodigestive tract endoscopy in case of tumors at or above the tracheal bifurcation) (Fig. 10.3a), and a CT scan (CAT scan) of chest and abdomen. In candidates for surgical resection endoscopic ultrasound has to be added to evaluate the T (and N) stage of the tumor (Fig. 10.3b); an esophagogram can be performed to assist in the planning of the surgical procedure. When available, positron emission tomography (PET) may be helpful in identifying otherwise undetected distant metastases or in diagnosis of suspected recurrence. PET/CT is preferred over PEt alone. In locally advanced (T3/T4) AC of the EGJ infiltrating the anatomic cardia, laparoscopy can rule out peritoneal metastases.
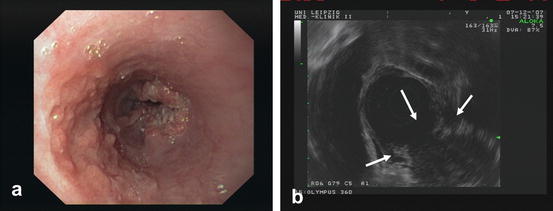

Fig. 10.3
SCC of the esophagus (a) endoscopic view and (b) endosonographic view
10.9 Treatment
Primary interdisciplinary planning of the treatment is mandatory. The main factors for selecting the type of primary therapy are tumor stage and location, histological type and the medical condition as well as the requests of the patients.
10.9.1 Endoscopic Treatment
Local endoscopic resection ± thermal ablation (PDT, BARRX) is indicated for tumors restricted to the mucosa with a size <2 cm and Barrett esophagus with low-grade or high-grade dysplasia. Endoscopic mucosal resection (EMR) is a procedure where the inner lining of the esophagus is removed with instruments attached to the endoscope in “piece meal technique”. Endoscopic submucosal dissection (ESD) of tumors has improved the success rate of “en bloc resection” but is still technically difficult for large lesions. After the abnormal tissue is removed, patients take drugs called proton pump inhibitors to suppress acid production in the stomach. This can help keep the disease from returning. Endoscopic therapy is highly effective and safe for patients with mucosal AC, with excellent long-term results. In an almost 5 year follow up of 1,000 patients treated by endoscopic resection, there was no mortality and less than 2 % had major complications [16]. Photodynamic therapy (PDT) is a method that can be used to treat tumor remnants after local endoscopic resection. PDT alone for early and late stage esophageal cancer has been abandoned due to a lack of acquisition of histology samples, limitation of effectivity to the tumor surface, and a high rate of postprocedural strictures. For this technique, a light-activated photosensitizer (porfimer sodium (Photofrin™) is injected into a vein. Over the next couple of days, the drug is more likely to enrich in cancer cells than in normal cells. A special type of laser light is then focused on the cancer through an endoscope. This light causes apoptosis inside the cancer cells. Instead of PDT radiofrequency ablation (RFA) can be used which rarely causes strictures or bleeding in the esophagus and induces a high rate of complete remission [17–19]. In this procedure, a balloon containing many small electrodes (BARRX™) is passed into an area of tumor through an endoscope. The balloon is then inflated so that the electrodes are in contact with the inner lining of the esophagus. Then an electrical current is passed through it, which kills the cells in the lining by heating them. Over time, normal cells will grow in to replace the tumor cells. Endoscopy (with biopsies) then is done periodically to watch for any further changes in the lining of the esophagus. In case of a non-operable tumor situation endoscopic metal stent placement is a proper solution to resolve dysphagia (Fig. 10.4). Stent placement is also important to treat certain surgical complications (see Sect. 10.9.2.1).
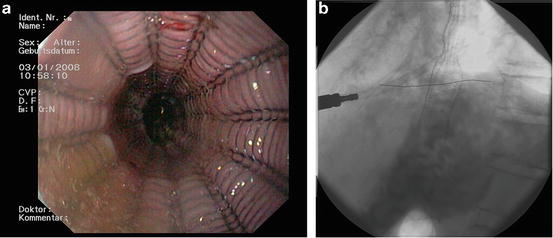

Fig. 10.4
AC of the esophagus- stent placement (a) endoscopic view and (b) X-ray
10.9.2 Surgery
Surgery is regarded as standard treatment only in carefully selected operable patients with localized tumors. T1/T2-tumors without metastases are suitable for primary surgery, in most of the cases subtotal en-bloc-esophagectomy with two field lymphadenectomy is preferred. For localized disease with suspected lymph node involvement (N1-3) preoperative therapy is recommended for AC. Transthoracic esophagectomy with two-field lymph node resection and a gastric tube anastomosized in the left neck is recommended for intrathoracic SCC. If the stomach has been removed in a previous operation a colon segment can be interposed. Debates continue about minimally invasive techniques that should reduce postoperative complication rates and recovery times. No standard treatment can be identified for carcinomas of the cervical esophagus. The extent of surgery in AC of the mid-to-distal esophagus or AC of the gastric cardia is still a matter of debate since one randomized study showed no significant improvement in long-term survival for extended transthoracic compared with transhiatal resection (in the case of a transhiatal resection lymph nodes of the middle and lower mediastinum are spared) [20, 21]. However, compared with limited transhiatal resection extended transthoracic esophagectomy for AC of the mid-to-distal esophagus showed an ongoing trend towards better 5-year survival. Moreover, patients with a limited number of positive lymph nodes in the resection specimen seem to benefit from an extended transthoracic esophagectomy. Another technique for AC of the gastric cardia is the Merendino procedure. This approach is suitable for patients without suspicion of lymph node involvement since lymph nodes of the middle and lower mediastinum and several lymph nodes of the lesser curvature of the stomach are spared. Best results are achieved by surgeons who have the highest numbers of esophageal cancer patients. A nationwide Swedish population-based study figured out a statistically significant reduction in 3-months mortality for surgeons with high annual or cumulative operation volume [22]. These results were similar to an earlier American study by Birkmeyer et al. [23]. Interestingly, there was no independent association between annual hospital volume and overall survival, and hospital volume was not associated with short-term mortality after adjustment for hospital clustering effects. This was different to the earlier American study by Birkmeyer et al. [24]. Patients who do respond poorly to neoadjuvant chemotherapy may benefit from a salvage operation [25], especially if R0-resection can be achieved [26]. In general, the most important prognostic factors of esophageal surgery seem to be curative (R0-) resection and extended lymphadenectomy.
10.9.2.1 Complications of Surgery
Typical complications of esophectomy are damage to the recurrent laryngeal nerve, tracheobronchial lesions, leakage or stenosis of anastomosis, necrosis of colonic interponate, pyloric spasm, and chylotharax. Esophageal stent therapy for approximately 4 weeks is recommended in the case of tracheobronchial lesions or leakage of anastomosis. Major defects with mediastinitis or necrosis of interponate require rethoracotomy. The surgeon is one of the most important prognostic factors.
10.9.3 Neaoadjuvant and Perioperative Chemotherapy
T3/T4 and T1-2 N+ AC tumors should be primarily treated with neoadjuvant chemotherapy or chemoradiation (see Sect. 10.9.5) in order to increase chance of curative (R0)-resection. A recent meta-analysis including ten studies and 2,062 randomized patients showed a significant improvement in overall survival (OS) after neoadjuvant chemotherapy, with a relative risk reduction of 13 % (HR 0.87; 95 % CI 0.79–0.96; p = 0.005), resulting in a 2 year survival benefit of 5.1 %. Whereas this difference was not significant for patients with SCC (HR 0.92; 95 % CI 0.81–1.04; p = 0.18) it was highly significant for patients with AC (HR 0.83; 95 % CI 0.71–0.95; p = 0.01) [27]. However, in Japan, neoadjuvant chemotherapy for SCC with cisplatin/5–fluorouracil is still regarded as standard treatment and can not be replaced by adjuvant chemotherapy with cisplatin/5–fluorouracil [28]. Perioperative chemotherapy of distal esophageal and EGJ cancer was first established with the phase III UK-MAGIC-study, which was published in 2006 in the New England Journal of Medicine and primarily designed for stomach cancer patients [29]. In this trial a perioperative ECF (epirubicin/cisplatin/5–fluorouracil) regimen (n = 250) decreased tumor size and stage and significantly improved progression-free survival (PFS) (HR 0.66; 95 % CI 0.53–0.81; p < 0.001) and overall survival (OS) (HR 0.75; 95 % CI 0.6–0.93; p = 0.009) in comparison to surgery alone (n = 253). Cisplatin/5-fluorouracil regimen as an alternative in this setting (distal esophageal, EGJ and stomach cancer) was published 5 years later derived from the results of the French FFCD multicenter phase III trial (n = 113 for perioperative chemotherapy and n = 111 for surgery alone) [30]. This trial showed a significantly increased curative resection rate, disease-free survival (DFS) (HR 0.65; 95 % CI 0.48–0.89; p = 0.003) and OS (HR 0.69; 95 % CI 0.5–0.95; p = 0.02). Interestingly, in these trials only 49.5 % respectively 50 % of patients who completed preoperative chemotherapy and surgery underwent postoperative chemotherapy as planned in the treatment protocol. This was mainly due to disease progression or early death, patient choice, postoperative complications, problems with the Hickman catheter, previous toxic effects, lack of response to preoperative treatment, and worsening coexisting diseases underlining the higher impact of preoperative chemotherapy on survival data.
10.9.4 Neoadjuvant Radiation
Neoadjuvant radiation was evaluated in six randomized fully published studies. Clinical response was detected in about one third of patients, however there was no significant advantage in survival. Two studies even reported a decreased OS after neoadjuvant radiation. A meta-analysis comprising 1,147 patients with mostly SCC from five randomized studies concludes that neoadjuvant radiation results in a 11 % relative risk reduction for the endpoint death (HR 0.89; 95 % CI 0.78–1.01) [31]. Difference in survival was 3 % after 2 years and 4 % after 5 years. This result was statistically non-significant (p = 0.062).
10.9.5 Neoadjuvant Chemoradiation
Preoperative chemoradiation with cisplatin/5–fluorouracil regimen and 41.4–45 Gy in 1.8 Gy fractions is recommended in T3-4 AC and SCC tumors and T1-2 N+ AC tumors. It is suggested, however, that preoperative chemoradiation will also increase post-operative mortality rates. In cases of response to neoadjuvant chemoradiation (SCC) further continuation results in equivalent OS compared with surgery alone, albeit that the non-operative strategy is associated with a higher local tumor recurrence (see Sect. 10.9.7). Bimonthly FOLFOX instead of the cisplatin/5-fluorouracil regimen does not improve PFS and has similar toxicities according to a large randomized phase III study (AC and SCC, PRODIGE 5/ACCORD 17 trial, ASCO 2012, LBA 4003 and Lancet Oncol 2014; 15: 305-314). The large phase III CROSS study from the Netherlands established a neoadjuvant carboplatin/paclitaxel/radiation regimen [32]. Two-hundred-seventy five patients (75 %) had AC, 84 (23 %) had SCC, and 7 (2 %) had large-cell undifferentiated carcinoma. Complete resection (R0) was achieved in 92 % of patients in the chemoradiation-surgery group versus 69 % in the surgery group (p < 0.001). Postoperative complication rate was similar in the two treatment groups, and in-hospital mortality was 4 % in both. Median OS was 49.4 months in the chemoradiation-surgery group versus 24.0 months in the surgery group (HR 0.657; 95 % CI 0.495–0.871; p = 0.003). After 24 months chemoradiation reduced local recurrence rate from 34% to 14% (p<0.001; Oppedijk V., JCO 2014; 32: 385-91). In contrast, the FFCD9901 phase III study did not show any benefit for neoadjuvant chemoradiation with 5-fluorouracil and cisplatin for early tumor stages UICC I and II (Mariette C., JCO 2014; 32: 2416-22). Finally, two meta-analyses of older randomized controlled trials for neoadjuvant chemoradiation showed a clear benefit in terms of OS in comparison to surgery alone, especially for patients with AC [27, 33]. In detail, the meta-analysis by Jin et al. comprised 11 randomized controlled trials from 1992 to 2008 including 1,308 patients [34–44]. The meta-analysis by Sjoquist et al. included 17 randomized controlled trials from their previous meta-analysis and 7 further studies. Twelve were randomized comparisons of neoadjuvant chemoradiation versus surgery alone (n = 1,854) [32, 34–42, 45, 46], nine were randomized comparisons of neoadjuvant chemotherapy versus surgery alone (n = 1,981), and two compared neoadjuvant chemoradiation with neoadjuvant chemotherapy (n = 194) in patients with resectable esophageal carcinoma. One factorial trial included two comparisons and was included in analyses of both neoadjuvant chemoradiation (n = 78) and neoadjuvant chemotherapy (n = 81).
10.9.6 Adjuvant Chemoradiation
In the pivotal Intergroup-0116 phase III trial by Macdonald et al. adjuvant chemoradiation (without preoperative chemotherapy) improved both DFS (HR 1.52; 95 % CI 1.23–1.86; p < 0.001) and OS (HR 1.35; 95 % CI 1.09–1.66; p = 0.005) in curatively resected patients with mainly gastric and EGJ AC [47]. Updated results from last year, confirmed that adjuvant chemoradiation (45 Gy radiation dose) remains a rational standard therapy for curatively resected gastric and EGJ cancer with primaries T3 or greater and/or positive nodes (n = 559 in the study) at least in the United States where D2 resection is less common than in Europe or Japan [48]. For this reason, the Intergroup-0116 study was criticized in Asia and Europe because a majority of patients received less than a D1 lymph node dissection at surgery, whereas fewer than 10 % underwent the more extensive D2 resection. This gave way to speculation that postoperative chemoradiation simply compensated for inadequate surgery. Although significantly fewer local and regional recurrences were found in the chemoradiation group the absolute number of local recurrences was to small to draw definitive conclusions. However, a Danish phase II study examining only patients with EGJ AC recently confirmed the Intergroup-0166 results (116 patients were treated with adjuvant chemoradiation) [49]. Median time of survival was prolonged by 10 months in favour of those who received chemoradiation.
10.9.7 Definitive Chemoradiation
Selected unfit patients with localized tumors not considered for surgery can be treated with curative intent by combined chemoradiation. Otherwise, principles of palliative therapy are recommended for these patients. A randomized controlled phase III study from the United States (RTOG trial 85-01) clearly demonstrated superiority of chemoradiation in comparison to radiation alone in patients with SCC and AC [50]. However, chemotherapy could be administered as planned in only 89 (68 %) of 130 patients (10 % had life-threatening toxic effects with combined therapy vs. 2 % in the radiation only group). Four courses of cisplatin/5–Fluorouracil combined with radiation doses of 50.4 Gy in fractions of 1.8 Gy are regarded as standard in the USA. Increased radiation doses up to 60 Gy in fractions of 1.8–2.0 Gy are recommended in parts of Europe and Japan. Michael Stahl and colleagues compared chemoradiation (etoposide and cisplatin, 40 Gy) followed by surgery (arm A, n = 86) with definitive chemoradiation (60 Gy) (arm B, n = 86). OS was equivalent in both SCC groups, local PFS was better in arm A (HR 2.1; 95 % CI 1.3–3.5; p = 0.003), but treatment related mortality less in arm B (3.5 % vs. 12.8 %, p = 0.03) [51]. These results were confirmed by a similar randomized French trial (259 patients were randomly assigned) using 5–fluorouracil and cisplatin as combination partners for radiation (only SCC patients) [52]. Median survival time was 17.7 months in the surgery group vs. 19.3 months in the definitive chemoradiation group. A third prospectively randomized study from Hong Kong (81 patients were randomly assigned) demonstrated a remarkable 5-year survival rate of 48.6 % for the definitive chemoradiation (5–fluorouracil/cisplatin/50–60 Gy) group and a trend to improved 5-year survival in node-positive disease (only SCC patients) [53]. In a recent study presented at ASC0 2012 (PRODIGE 5/ACCORD 17 trial; Hong TS et al.; LBA 4003 and Conroy T., Lancet Oncol 2014; 15: 305-314) patients with non-operable localized esophageal carcinoma (85 % SCC, 15 % AC) were randomized to two different chemoradiation protocols. Radiation dose was 50 Gy in both arms. Patients in A received six cycles of FOLFOX (5–fluorouracil/leucovorin/oxaliplatin) every 2 weeks and patients in arm B four cycles of 5–fluorouracil/leucovorin/cisplatin every 3 weeks. PFS (9.7 month vs. 9.4 month), the primary study endpoint, and OS survival (20.2 months vs. 17.5 months) were similar in both arms. Therefore, a radiation protocol with FOLFOX may be a good alternative in patients with renal insufficiency or reduced general condition. Addition of epithelial derived growth factor receptor (EGFR) 1 inhibitor cetuximab to a capecitabine/cisplatin/radiation backbone did result in greater toxicity, lower rate of completion of standard therapy and significantly worse survival (22 months vs. 25 months; p = 0.043) in patients with locally advanced SCC (73 %) or AC (27 %) as demonstrated by a recent large UK study (SCOPE-1, NCT00509561) [54]. Docetaxel/cisplatin/radiation combination is feasible too, as demonstrated in a Korean phase II study (36 SCC patients) [55]. Moreover, carboplatin/paclitaxel/radiation as in the neoadjuvant setting (see above) is another option (Honing J., Ann Oncol 2014; 25: 638-643). In a recent meta-analysis of three randomized studies definitive chemoradiation in patients with SCC did not demonstrate any survival benefit over other curative strategies, but treatment-related mortality rates were lower (HR 7.60, p = 0.007) [56]. In addition, a recent analysis from the American National Cancer Database unveiled that OS was lower for patients with stage II/III disease of either histologic subtype treated with chemoradiation alone as compared with surgery plus chemoradiation (p < 0.001) [57]. A study from Korea suggested vascular endothelial growth factor (VEGF) as positive predictive factor and cyclooxygense-2 (COX-2) as negative prognostic factor for OS in patients with SCC after definitive chemoradiation [58]. Improvement of definite chemoradiation for locally advanced disease is a focus of current research. Proton-beam therapy (PBT) and intensity modulated radiation therapy (IMRT) are both forms of radiation therapy that are designed to treat a specific area of the body while affecting as little of the surrounding normal tissue as possible. PBT is a newer technology that is designed to further reduce the amount of radiation that affects the surrounding normal tissue. A particle accelerator is used during treatment to hit the tumor with a beam of protons. As a result, DNA damage of cells is induced by these charged particles, ultimately resulting in cell death or decrease of cell proliferation. Since tumors show a high rate of cells division and a reduced rate of cell repair they are particularly vulnerable to attacks on DNA. Protons have little lateral side scatter in the tissue due to their relatively large mass. The beam stays focused on the tumor shape, does not broaden much, and causes only low-dose side-effects to surrounding tissue. IMRT, which is less expensive, comprises an advanced mode of high-precision radiotherapy that uses computer-controlled linear accelerators (3-D computed tomography (CT) or magnetic resonance images (MRI) are used for planning) to deliver precise radiation doses to a malignant tumor or specific areas within the tumor. The radiation dose can be more precisely adjusted to the three-dimensional shape of the tumor by modulating—or controlling—the intensity of the radiation beam in multiple small volumes. Using IMRT, higher radiation doses and combinations of multiple intensity-modulated fields coming from different beam directions can be focused to regions within the tumor while the dose to surrounding normal critical structures can be minimized.
10.9.8 Palliative Chemotherapy
10.9.8.1 First Line Chemotherapy
In the past decades, there was not much improvement in the outcome and survival of advanced esophageal cancer (M1) due to the lack of effective chemotherapy agents. In SCC, the value is even less proven than in AC. The traditional chemotherapy drugs to treat esophageal cancer include 5–fluorouracil and cisplatin and the combination of them results in a 25–35 % RR in both first-line and second line treatment [59]. Unfortunately, the main side effect of cisplatin is renal toxicity. The peak age of esophageal cancer patients is 65–70 years and many of them have simultaneously other diseases such as hypertension, diabetes, and chronic kidney disease which cause varying damages of renal function and limit the use of cisplatin in these patients. Therefore, it is urgent and crucial to seek an alternative less toxic treatment regimen. Due to high response rates in Asian patients a combination of cisplatin/oral fluoropyrimidine S–1 was compared with cisplatin/infusional 5-FU in patients with advanced gastric or EGJ AC (FLAGS trial) [60]. One thousand fifty-three patients were stratified and the primary end point was superiority in OS from cisplatin/S–1. Although this goal was not met in the cisplatin/S–1 arm (HR, 0.92; 95 % CI, 0.80–1.05; p = 0.20), significant safety advantages were observed in the cisplatin/S–1 arm compared with the cisplatin/infusional fluorouracil arm for the rates of grade 3/4 neutropenia (32.3 % vs. 63.6 %), complicated neutropenia (5.0 % vs. 14.4 %), stomatitis (1.3 % vs. 13.6 %), hypokalemia (3.6 % vs. 10.8 %), and treatment-related deaths (2.5 % vs. 4.9 %; p < 0.05). 5–fluorouracil can also be replaced by oral capecitabine [61] (XP regimen) and cisplatin by oxaliplatin [62], based on phase II studies. Dual replacement was successful, too [63, 64]. Regarding toxicity, FLO (5–fluorouracil/leucovorin/oxaliplatin) seems to be less toxic than FLP (5–fluorouracil/leucovorin/cisplatin) according to a phase III study including mostly gastric cancer patients but also patients with EGJ tumors [65]. Paclitaxel plus cisplatin regimen is another promising treatment of esophageal cancer and has been proved effective at phase II level [66]. This combination has become a standard treatment of esophageal cancer, especially of SCC. However, the lower solubility of paclitaxel limited its direct intravenous use. To solve this problem, paclitaxel must be injected with an additional surfactant polyoxyethylene castor oil. Polyoxyethylene castor oil paclitaxel could induce high incidence of acute hypersensitivity reactions, i.e. severe allergic reactions, kidney damage, neurotoxicity, and cardiovascular toxicity which is characterized by axonal degeneration and demyelination. Though proper preventive treatment will greatly reduce the incidence of allergy, there is still a small number of patients who have allergic reactions. In addition, paclitaxel or docetaxel can be combined with capecitabine [67–69]. In AC patients with a good general condition triplet regimens, such as ECF (epirubicin/cisplatin/5–fluorouracil), ECX (epirubicin/cisplatin/capecitabine), EOF (epirubicin/oxaliplatin/5–fluorouracil), and EOX (epirubicin/oxaliplatin/capecitabine), or DCF (docetaxel/cisplatin/5–fluorouracil)/DCX (docetaxel/cisplatin/capecitabine), and DCC (docetaxel/carboplatin/capecitabine) are even more effective regarding response rate, however toxicity is markedly increased [70–74].
10.9.8.2 Second Line Chemotherapy
In case of treatment failure or relapse second line treatment may be indicated in patients who are still fit enough to tolerate chemotherapy. These are approximately 40 % of patients who received first line treatment. Unfortunately, currently there is only scarce data from prospective phase II studies dealing with this group of patients.
Vinorelbine [75], docetaxel [76, 77], paclitaxel [78], and irinotecan [79] were investigated as monotherapy. Due to the low number of study participants and low RR in these studies none of the substances could be recommended for second line therapy. However, a recently presented randomized study (Cougar-02, Ford et al. 2013 Gastrointestinal Cancers Symposium, LBA4 and Lancet Oncol 2014; 15: 78-86) which compared docetaxel monotherapy with best supportive care (BSC) in patients with stomach (46 %), EGJ (34 %) and esophageal cancer (20 %) demonstrated that docetaxel significantly improves OS. Taxane-based combinations were tested in several prospective phase II trials including a combination of docetaxel plus capecitabine [69], docetaxel plus irinotecan [80, 81], docetaxel plus cisplatin [82], and docetaxel plus nedaplatin [83–86]. In the first three combination regimens, RR was still low and rate of hematologic toxicity high, e.g. severe neutropenia occurred in almost half of the patients receiving docetaxel plus capecitabine. Although hematologic and non-hematologic toxicity was relatively low with docetaxel plus nedaplatin combination these studies included only Asian patients making it difficult to interprete these results for Caucasians. In addition, RR was still low, too. In view of the high activity of DCF-type regimens in first line treatment the combination of docetaxel, cisplatin, and 5-fluorouracil was investigated in second line setting, too [87, 88]. Whilst dose reduction of all drugs in the first study resulted in lower RR, increased dose in the second study resulted in remarkable hematologic toxicity. Finally, only a single non-taxane combination regimen consisting of mitomycin, ifosfamide, and cisplatin was tested [89]. Although toxicity rate was acceptable, RR was low, too.
10.9.9 Supportive Palliative Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree