Fig. 46.1
Histogenesis of ovarian cancer
Table 46.1
Histogenetic classification of ovarian neoplasms
Neoplasms derived from coelomic epithelium |
Serous tumor |
Mucinous tumor |
Endometrioid tumor |
Mesonephroid (clear cell) tumor |
Brenner tumor |
Undifferentiated carcinoma |
Carcinosarcoma and mixed mesodermal tumor |
Neoplasms derived from germ cells |
Teratoma |
Mature teratoma |
Solid adult teratoma |
Dermoid cyst |
Struma ovarii |
Malignant neoplasms secondarily arising from mature cystic teratoma |
Immature teratoma (partially differentiated teratoma) |
Dysgerminoma |
Embryonal carcinoma |
Endodermal sinus tumor |
Choriocarcinoma |
Gonadoblastoma |
Neoplasms derived from specialized gonadal stroma |
Granulosa-theca cell tumors |
Granulosa tumor |
Thecoma |
Sertoli–Leydig tumors |
Arrhenoblastoma |
Sertoli tumor |
Gynandroblastoma |
Lipid cell tumors |
Neoplasms derived from non-specific mesenchyme |
Fibroma, hemangioma, leiomyoma, lipoma |
Lymphoma |
Sarcoma |
Neoplasms metastatic to the ovary |
Gastrointestinal tract (Krukenberg) |
Breast |
Endometrium |
Lymphoma |
The World Health Organization (WHO) histologically classified epithelial ovarian tumors in histopathological subtypes, serous, endometrioid, clear cell, mucinous, Brenner (transitional cell), mixed epithelial tumors, undifferentiated and unsorted, histopathologic subtype and grade characteristics architectural, nuclear atypia and mitotic index have prognostic significance. No single classification, universally accepted [5].
46.2 Epithelial Ovarian Cancer Pathogenesis
The EOC is the term used for malignant tumors arising in the ovary with involvement of the uterine tube and peritoneum; these epithelial neoplasms are divided into two groups according to the source, either ovaries or oviducts [10]. The first group of cancers that originate in the ovary, are histopathological subtypes: endometrioid, mucinous, clear, borderline and low-grade serous cells, some develop endometriosis, benign ovarian disease affecting mainly endometrioid subtypes and clear cell [11].
Müller inclusions in the ovarian cortex are another source of primary ovarian carcinomas and have been implicated in the development of mucinous and serous neoplasms lesser extent, the morphological evidence is the gradual spectrum of changes observed in the ovary, deriving cortical inclusions cistadenofibromas, carcinomas and serous borderline low grade. Oviducts, uterus, cervix and upper third of the vagina are derived from Mullerian ducts or paramesonephric, while the ovary develops from primordial germ cells surrounding the epithelial surface [6]. The Müller primary neoplasms in the ovary originating in the cells acquired during the reproductive years, including transport of epithelial cells of the endometrium or uterine tubes, even also the Müllerian metaplasia of the ovarian surface epithelium [12].
The second group consists of pelvic serous carcinomas extrauterine, that high grade and poor prognosis traditionally are considered carcinomas of primary ovarian, some carcinomas of the uterine tubes and peritoneal carcinomas, the characteristics of these carcinomas are rapidly progressing, extraovarian disease at diagnosis and no known precursor lesion.
Current evidence suggests that many of these neoplasms originate from the uterine tube or tubes and refer to the term “ectopic pelvic serous carcinoma” through the origin in ovarian Mullerian duct or elsewhere in the peritoneal cavity.
The EOC type 1 cancers are low-grade and good prognosis include low-grade serous, endometrioid, mucinous, clear cell and malignant Brenner tumors, these tumors are characterized by mutations in KRAS, BRAF, ERBB2, PTEN, PIK3CA and ARID1A and are genetically stable, these mutations occur early in evolution and is also noted in borderline tumors and endometriosis, are developed in phases benign precursor lesions (such as borderline tumors) to malignant lesions. By contrast, in type II EOC. No precursor lesion, are high grade, aggressive and include serous high-grade endometrioid high-grade, malignant mixed mesodermal tumors and undifferentiated tumors [13]; frequently associated with mutations of TP53; serous high-grade 97 % and 20 % of these have BRCA1/2 genes due to the combination of germline and somatic mutations and most of the tumors of the ovary and peritoneal serous high originate in the fimbriae of the uterine tube or tubes (neoplasia intraepithelial tubal serosa) and then this malignant cells metastasize to the ovaries and peritoneal cavity [14–18] Table 46.2.
Table 46.2
Epithelial ovarian cancer types
Type I low risk | Type II high risk |
|---|---|
Grade I | Grade II and III |
Histopathological no clear cell type | Clear cell |
Capsule integrates | Tumor growth through the capsule |
No surface excrescences | Excrescences on the surface on the surface excrescences |
No ascites | Ascites |
Negative cytology | Cytology positive |
No break or rupture during surgery | Previously rupturing the surgery |
No dense adhesions | Adhesions dense |
Diploid | Aneuploidy |
The disease-free (DFS) and survival with ECO type I are were longer than in patients with EOC type II (P < 0.001 and P < 0.001, respectively) after optimal cytoreduction, the DFS and survival are shorter in patients with CA-125 nivles 11–35 U/ml and in type II EOC with CA-125 levels straight 10 U/mL or less and type I EOC [19].
46.3 Histopathology Epithelial Ovarian Cancer
The histological type of ovarian cancer, uterine tube or tubes and peritoneum are frequently epithelial, ovarian cancer are considered as a single entity, but consists of a heterogeneous group of neoplasms with multiple histopathological subtypes. Current management of these tumors depends on factors such as tumor grade and stage, but it is important to accurately subclassified these tumors, as each is a biologically different disease with different epidemiological factors and genetic risk precursor lesions, patterns propagation, molecular biology, response to treatment and prognosis, as new therapies are developed, it is essential to determine which subtypes of ovarian carcinomas, uterine tubes or tubes and peritoneal respond to treatment modalities [5, 7–9, 20].
At present, based on the histopathology, immunohistochemistry and molecular genetic analysis of the five major subtypes of ovarian epithelial carcinomas, uterine or fallopian tuba and peritoneal ratios are [5, 9]: high-grade serous carcinoma (70–80 %), endometrioid carcinoma (10 %), clear cell carcinomas (10 %), mucinous carcinoma (3 %), low-grade serous carcinoma (<5 %). Now accepted that the high-grade serous carcinoma (HGSC) and low-grade serous carcinoma (LGSC) are different neoplasms with different molecular pathogenesis, although both originate from precursors of the uterine tube or tubes; intraepithelial neoplasia tubal serosa to carcinoma in case endosalpingiosis HGSC and/Mullerian if LGSC [21, 22]. Transitional cell carcinoma had been historically included as a distinct subtype, although recent molecular evidence supports this as a subset of serous carcinoma.
There invasive or borderline for some subtypes of ovarian carcinomas neoplastic counterparts, as neighboring or Borderline malignancies. Immunohistochemical profiles and molecular biology differ between prognostic subtypes histopatplógicos and sons: the high-grade serous carcinomas typically have mutations in the BRCA genes TP53. The low-grade serous carcinomas often have KRAS mutations and BRAF [23].
46.3.1 High-Grade Serous Carcinoma
The HGSC is the most common type of ovarian cancer and represents 70–80 % of all malignant ovarian tumors, the peak age range is 45–65 years, with an average of 57 years. Most HGSC diagnosed at advanced stage (stage III or IV) are generally poor prognosis: it is rare that the HGSC this confined to the ovary at diagnosis (<10 %), the HGSC vary in size from microscopic to larger 20 cm in diameter, the outer surfaces are smooth or have friable surface papillae. The mass is typically multilocular cystic serous or bloody fluid and soft friable papillary excrescences. Other areas are usually solid, or soft to firm, depending on the tumor stroma. Hemorrhage and necrosis are often present, metastases are often found throughout the peritoneal cavity and omentum, as firm nodules of different sizes together in large masses, often simulates a cake omentum, omentum 25 % seems to be macroscopically normal, but you’re concerned microscopically, the HGSC has a variety of architectural patterns including papillary complex, glandular, microcystic and solid, the HGSC infiltrate, destroy and/or replaces the normal stroma; bodies psammoma are present, but rare time are as numerous as the LGSC [12, 24–26]. The key feature of HGSC, regardless of architectural pattern, is the marked cytologic atypia with prominent mitotic activity. Atypical nuclei are hyperchromatic with a threefold or greater variation in nuclear size and giant cell tumors are common, with high mitotic index ≥12 mitoses per 10 high power fields (HPF), if the mitotic index is low, consider LGSC u other diagnosis, the cytoplasm of cells with HGSC is focal clear cell change, but care must be taken with the mixed HGSC diagnosis and clear cell carcinoma, as these tumors have different molecular etiologies and a mixed tumor containing carcinoma serous serous and clear cell is rare [12].
The HGSC usually expressed firmly WT -1, estrogen, PAX -8 in most cases and so diffuse p53 and p16 without express HNF -1 beta and calretinin, have high Ki67 proliferation index. Germline mutations in BRCA1 or BRCA2 in 10 % and women with these germline mutations are identified at risk of 30–50 % of developing ovarian cancer, mainly HGSC, at 70 years of age, 50–80 % of HGSCs, regardless of the status of BRCA germline, have mutations in tumor gene protein p53 (TP53), and loss of function mutations in TP53 HGSC 80 % identify and identified the putative precursor lesion of many HGSCs the serous intraepithelial neoplasia and a few other specific genetic mutations have been identified in HGSC, though, changes in gross chromosomal instability and DNA copy number are consistently HGSC; mutations in the PTEN gene and PI3CA have also been reported, with lower frequency (<10 %) [5, 12, 26–32].
46.3.2 Low Grade Serous Carcinoma
The LGSC is rare and represents less than 5 % of all cases of ovarian carcinomas [12, 24, 25], are diagnosed at an advanced stage and the long-term prognosis is poor, they are relatively slow growing insensitivity to chemotherapy (Qt) based on platinum [12, 26, 27]; are often coupled with a serous borderline non-invasive or component, probably represents the progression of neoplasia serous borderline. The LGSC is often indistinguishable from HGSC or serous borderline, the LGSC are solid and cystic with numerous friable papillary excrescences within cysts or on the surface, with less haemorrhage and necrosis and extraovarian implants are typically firm sandy due to stromal reaction and the abundant formation of psammoma bodies; histopatplógicamente distinguished from serous borderline tumors by the presence of destructive stromal invasion. The three main patterns of invasion are; stromal infiltration by individual cells and small groups of cells, stromal infiltration by small nests of epithelial cells (micropapillary pattern) and stromal infiltration by large papillae with fibrovascular comprehensive nuclear center bordered by neoplastic cells (macropapilar pattern), the LGSC consists of small papillae lined by neoplastic cells with uniform nuclei with less variability in size three times, the uniformity of nuclear size is the distinguishing feature of HGSC LGSC [27, 28], another distinctive feature of LGSC is that it has less than HGSC mitotic activity, with <12 mitoses per 10 high power fields (HPF), although the inferior LGSC mitotic index demonstrates even 11–10 per HPF, useful for differentiation of HGSC feature, another feature is a distinctive hyalinized stroma with numerous psammoma bodies; LGSC immune phenotype is similar to both serous borderline tumors and HGSC, but with two important differences: the LGSC has low Ki67 proliferation rates (corresponding to low mitotic rate) and wild-type or weak expression of p53, the latter is not statistically significant prediction of p53 mutation analysis and identification may be serous carcinoma of low or high grade printing without voiding the histopathological [2, 5, 12], the LGSC express WT1, Receivers estrogen and progesterone, are negative for HNF-1ß and calretinin, often they have mutations in BRAF and KRAS, rather than p53 or BRCA 1/2, as HGSC [2, 3, 5], the LGSC and serous borderline tumors do not show generalized prominent or instability or chromosomal alterations in DNA copy as seen in HGSC; indicate two-way serosa carcinogenesis; HGSC typical pathway, where mutations in p53 plays an important role and the second common path of tumors low-grade serous, KRAS and BRAF which play a prominent role; serous borderline tumors implicated as the precursor lesion of low-grade serous carcinoma and support the hypothesis that low-grade serous carcinomas alos not progress HGSC [28]. These molecular differences affect treatment in the future as new agents are used to attack the KRAS and BRAF pathways in LGSC, that would be resistant to platinum-based Qt [12, 24–33].
46.3.3 Endometrioid Carcinoma
Endometrioid ovarian carcinoma represents 10 % of all ovarian carcinomas and occurs most frequently in women aged 40–50 years, mean age 56 years [5, 12], occurring in early stage (unlike serous carcinomas) [34], have a better prognosis and are relatively sensitive to Qt (unlike LGSC), which contributes to better prognosis compared to other subtypes of ovarian carcinoma. Endometrioid adenocarcinoma primary ovário is typically low grade and high grade are morphologically and molecularly indistinguishable from HGS, immunophenotypic profiles and genes suggest that endometrioid carcinoma high grade is a different type of tumor is a subtype of HGSC [29]; endometrioid ovarian carcinoma arises is associated or endometriosis (42 % of the patients have evidence of ovarian endometriosis or pelvic) [5, 8, 9, 11, 12, 29–31, 35].
The endometriode ovarian carcinoma is associated with endometrial cancer in 15–20 % and histologically similar and cancer concurrent endometrium with metastases the ovary is high, although other possibilities are metastases from ovary to endometrial or ovarian neoplasms and primary endometrial simultaneous. Grossly, endometrioid carcinoma has a variable appearance, may be cystic or solid, with residual foci of endometriosis with the typical appearance of endometrioma or chocolate cyst with smooth outer surfaces and is generally limited to one ovary (the favors bilateral metastases endometrial cancer) [26]. Papillary excrescences views serous carcinomas are not present, although the growth is quite friable with areas of hemorrhage and necrosis; has grossly identifiable areas adenofibroma with endometrioid stroma separated by prominent cleft cysts firm are often in association with endometrioid carcinoma or borderline; histopathologically, endometrioid carcinoma of the ovary resembles that of endometrioid type of low-grade cancer. Most ovarian endometrioid carcinomas have a complex cribriform and/or architectural vellosoglandular glandular growth pattern with back to back or round or elongated glands with luminal smooth contours. The glands are typically lined by stratified columnar cells with scant eosinophilic cytoplasm and nuclei of low-grade intermediate. Mitotic figures are seen frequently; morulas contain the immature squamous cells, often are present within the tumor. Foci of cells with typical secretory changes are commonly identified and believed to be due to the effect of endogenous or exogenous progestogens, but occurs in the absence of hormone stimulation, when a sheet -shaped pattern of cell growth with a high degree nuclear is identified HGSC diagnosis must be considered. Endometriosis in the epithelium and endometrial stroma occurs frequently and it is unusual spectrum of lesions ranging from atypical endometriosis, endometrioid borderline neoplasia and endometrioid carcinoma. Endometrioid adenocarcinomas low degree express similar to endometrial endometrioid type cancer marker, is expressed vimentin, estrogen receptor and progesterone, PAX -8 and CA-125, are negarivos to the p16 and p53 or are expressed focally, are also negative for WT -1, calretinin and inhibin; endometrioid carcinomas have similar high HGSC profiles are diffuse and express p53, p16 and WT -1, indicating that the high-grade endometrioid carcinoma is a subtype of HGSC. Several mutations have been identified in genes endometrioid ovarian carcinoma; somatic mutation in CTNNB -1 (beta -catenin) and PTEN genes are genetic abnormalities identified endometrioid ovarian carcinomas [33]. PIK3CA mutations and ARID1A, with a component of a large multiprotein complex which behaves as tumor suppressor gene, are often observed and high levels [36] of microsatellite instability observed endometrioid ovarian carcinomas and this histopathologic subtype of carcinoma ovary is most common associated with Lynch syndrome [25, 34, 35].
46.3.4 Clear Cell Carcinoma
Clear cell carcinoma accounts for 5–10 % of all ovarian carcinomas are most commonly in perimenopausal women aged 40–50 years old [24, 25]. The clear cell carcinoma is more common in East Asia, of course without being be due to genetic or environmental, occurring in early stage (stage I or II) with relatively good prognosis due to the absence of distant metastases, but in advanced stage, have a worse prognosis than serous or endometrioid carcinoma, because the clear cell carcinoma is not as sensitive to platinum-based Qt as the other subtypes [34, 36] histopathological also is associated with increased risk of vascular thrombotic events and paraneoplastic hypercalcemia and associated or probably derived from endometriosis, tubas uterinas occlusion protects against the development of clear cell carcinoma of ovary to prevent retrograde menstruation and development of endometriosis [11, 35].
In addition to the association of clear cell carcinoma with endometriosis, clear cell carcinomas are often diagnosed as adenofibromas arising from clear cell [12, 34], 15–20 % of clear cell carcinomas have a dominant component and is a adenofibromatoso subgroup with clinicopathological features. Clear cell carcinoma of the ovary is often presented as a large mass, average size 15 cm. The cystic tumor uni or multilocular thick-walled with fleshy nodules yellowish protruding from the lumen of the cyst(s) and contain aqueous liquid or mucosa in the cyst(s) may also be solid or have to cut an area of honeycomb adenofibroma especially combined with clear cell or borderline clear cell neoplasia: neoplasms arising from endometriosis have characteristics of endometriomas, with chocolate brown liquid and thickened nodular area on the wall that represents the area of malignant transformation; show many different histopathological patterns that often occur together in the same neoplasm [5, 9, 12]. The most common patterns are solid, tubulo – cystic and papillary; leaves polyhedral cells with clear cytoplasm are separated by fibrous stroma characterize the solid pattern. The tubule-cystic pattern contains several tubules and cysts that can be mixed with other complex patterns. Papillary pattern is formed by varying complexity buds fibrous bordered by neoplastic cells. Both tubular- papillary cystic patterns neoplastic cells often assume an appearance, the core protruding from the cyst or lumen and the papillae; independently architecture pattern generally clear cell carcinomas often contain a prominent stroma hyalinized. Neoplastic cells often have edges of differentiated cells with nuclei of different sizes and shapes and have wide range of nuclear atypia, although there are usually areas with marked cytologic atypia, the epithelial lining of glands and cysts is plane, creating the appearance deceptively. Mitotic activity is prominent, variable, but lower than that observed in other epithelial ovarian carcinomas, lacking expression of both estrogen receptors and WT- 1. They may have a little expression of p53, although strong and diffuse expression noted in HGSC not normally identified. Clear cell ovarian normally express the hypoxia inducible factor 1alpha (HIF -1alpha), glypican -3, and nuclear – beta 1 (HNF -1 beta) factor hepatocyte. The HNF -1 beta appears to be a sensitive and specific marker of clear cell carcinoma of the ovary 82–100 % expressing this protein and, occasionally, others express epithelial ovarian carcinomas. Mutations in KRAS, PIK3CA and PTEN are reported in clear cell carcinoma of ovary [34, 35], as in endometrioid ovarian carcinoma, high levels of microsatellite instability observed in clear cell carcinoma of ovary and [21, 35, 37] associated with Lynch syndrome, also have identified mutations in ARID1A, similar to endometrioid carcinoma.
46.3.5 Mucinous Carcinoma
Mucinous carcinoma represents 3–4 % of cancers of primary ovarian, is more common in perimenopausal women aged 40–50 years old, have been reported in women reproductiva age and over 87 years old, most are identified early stage, usually stage I, of all types of ovarian mucinous tumors represent 10–15 %, 80 % are benign mucinous cystadenomas and most of the others are borderline mucinous tumors and most mucinous carcinomas metastatic ovarian often derived from the gastrointestinal tract mucinous carcinomas arise from primary ovarian mucinous borderline tumors and mucinous cystadenomas and borderline tumors and high grade; intraepithelial neoplasia and mucinous carcinoma are often seen in the same tumor [12, 23–26, 28, 32]. The primary ovarian mucinous tumor size range of 8–20 cm carcinoma, but can be much larger [12, 24, 26]. Usually it is cystic or solid, unilateral and confined to the ovary. The outer surface is usually smooth, without participation of the surface [32]. Ovarian mucinous neoplasms are bilateral involving the surface and not confined to the ovary are often a metastasis, usually in the gastrointestinal tract [12, 24–26, 28]. Mucinous carcinomas of primary ovarian not presented as peritoneal pseudomyxoma, though, this historically believed it was a result of the breakdown of primary ovarian mucinous neoplasm is now accepted that pseudomyxoma peritoneal is caused by metastasis to the ovary, often the primary carcinoma of the appendix and in rare cases of primary ovarian teratomas [12, 22]. Cells mucinous ovarian carcinoma resembles the bowel or pylorus endocervix, most of these tumors have gastrointestinal differentiation. The same histopathology seen in mucinous tumors borderline and the primary distinction between a mucinous carcinoma and mucinous tumor boundary is the presence of invasion. The tumors show glandular complex arrangements with areas of stromal invasion greater than 10 mm2 or 3 mm linear extension [12]. Two patterns of invasion of mucinous carcinomas have been described: infiltration and expansion. The invasion of destructive infiltrates stroma form destroys the glands, groups of cells, or individual cells randomly infiltrating stromal often accompanied desmoplastic stromal reaction. Infiltration of invasion of invasive carcinoma has a worse prognosis. By contrast, the expansive growth patternmshows no invasion os stroma, but the complex architecture of the glan back to back and brought minimum stroma causes invasive carcinoma and mucinous carcinoma with espansive growth, invasion equivalent in other es named EOC [12]; also classified as mucinous ovarian tumors with complex architectural pattern defined growth without invasion destructive invasion of the stroma is not identified, but is associated with favorable prognosis, these tumors are not realy invasive carcinomas. Mucinous ovarian carcinoma often expressed gastrointestinal markers, includindg CK20 and CDX2 plus CK2 expression [24–28]. Irregularly without express p16 expression estrogenic or progestational receptors, WT-1 and CA-125 [34–36]; immunohistochemistry is not useful for determining whether a mucinous ovarian tumor is a primary or metastatic ovarian neoplasm; over 75 % mucinous ovarian carcinomas have KRAS mutation [32, 33]. KRAS mutations are identical to those observed in mucinous cystadenomas and mucinous tumors borderline, supporting tumor progression model of mucinous cystadenoma borderline carcinoma, mucinous ovarian carcinomas express multiple mucin genes (MUC2, MUC3 and MUC17) which is characteristic mucinous carcinomas irrespective of the tissue of origin [24, 25, 32, 34–36].
46.3.6 Other Histopathological Subtypes
Transitional cell carcinoma is defined as epithelial neoplasm composed of elements similar to histologically benign urothelium lacking Brenner tumor component or borderline. It was believed that transitional cell carcinomas were examples of malignant Brenner tumors in the benign component was uncovered, but the molecular and immunohistochemical data showed that tumors previously classified as transitional cell carcinomas express the same phenotype and genetic mutations as HGSC [35]; currently transitional cell carcinoma is simply a subset of HGSC where the epithelium is morphologically similar to malignant urothelium. Carcinosarcoma also referred to as mixed Müller malignant tumor (MMMT) comprises give 2 % and 7.5 % of ovarian carcinomas are presented at an average age of 75 years tend to be large with plenty of hemorrhage and necrosis in advanced stage; Histologic features by mixture of malignant epithelial and stromal elements.
The most common malignant epithelial component resembles HGSC, but reported other subtypes and malignant stromal component usually contains rounded hyperchromatic nuclei; fusiforms cells with nuclear atypia and high mitotic index marked. Heterologous elements such as cartilage, osteoid and rhabdomyoblasts are commonly seen. Stromal components are often positive, at least focally to epithelial and like most carcinosarcomas endometrial, ovarian carcinosarcoma are monoclonal, suggesting that they are markers metaplastic carcinomas, are aggressive and behave similar to HGSC both propagation, response to platinum-based Qt and Forecast [13].
Undifferentiated carcinoma defined by the World Health Organization (WHO) as primary ovarian carcinoma with little or no differentiation is rare and probably represents the extreme HGSC spectrum often contains undifferentiated foci; expressing WT-1 and indicates most undifferentiated carcinomas are HGSC with little or no morphological differentiation [26]. Surgical staging of ovarian carcinoma FIGO 2014, Table 46.3.
Stage I: Tumor confined to ovaries | |
IA | Tumor limited to 1 ovary, capsule intact, no tumor on surface, negative washings |
IB | Tumor involves both ovaries otherwise like IA |
IC | Tumor limited to 1 or both ovaries |
IC1 | Surgical spill |
IC2 | Capsule rupture before surgery or tumor on ovarian surface |
IC3 | Malignant cells in the ascites or peritoneal washings |
Stage II: Tumor involves 1 or both ovaries with pelvic extension (below the pelvic brim) or primary peritoneal cancer | |
IIA | Extension and/or implant on uterus and/or fallopian tubes |
IIB | Extension to other pelvic intraperitoneal tissues |
Stage III: Tumor involves 1 or both ovaries with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes | |
IIIA | (Positive retroperitoneal lymph nodes and/or microscopic metastasis beyond the pelvis) |
IIIA1 | Positive retroperitoneal lymph nodes only |
IIIA1(i) | Metastasis ≤10 mm |
IIIA1(ii) | Metastasis >10 mm |
IIIA2 | Microscopic, extrapelvic (above the brim) peritoneal involvement ± positive retroperitoneal lymph nodes |
IIIB | Macroscopic, extrapelvic, peritoneal metastasis ≤2 cm ± positive retroperitoneal lymph nodes. Includes extension to capsule of liver/spleen |
IIIC | Macroscopic, extrapelvic, peritoneal metastasis >2 cm ± positive retroperitoneal lymph nodes. Includes extension to capsule of liver/spleen |
Stage IV: Distant metastasis excluding peritoneal metastasis | |
IVA | Pleural effusion with positive cytology |
IVB | Hepatic and/or splenic parenchymal metastasis, metastasis to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity) |
46.4 Risk Factors
The pathogenic mechanisms related to risk factors and the development of EOC is involved incessant ovulation, ovulation which causes trauma to the ovarian epithelium, leading to malignant transformation; oppositely suppression of ovulation by oral contraceptive use, pregnancy, lactation, decreases the incidence of epithelial ovarian cancer and persistent exposure to gonadotropins and high concentrations of estradiol are carcinogenic also ovarian tumors contain receptors for gonadotropin, however, a history of multiple pregnancy is associated with reduced risk of EOC and should be at increased risk of EOC, as they have higher levels of gonadotropins during their childbearing years with the highest incidence of double ovulations per menstrual cycle even, no relationship between serum levels of luteinizing hormone and the risk of ovarian cancer was demonstrated. Cancer of the fallopian tube or tubes also plays a role in the pathogenesis of the EOC and peritoneal cancer, where the risk factors in general are the same and are [38, 39] age, reproductive factors and hormonal, early menarche or Late menopause, nulliparity and other obstetrical factors, infertility, endometriosis, polycystic ovarian syndrome, hormone replacement therapy, intrauterine device, genetic factors, family history, inherited, hereditary cancers (Lynch syndrome), environmental factors (smoking, talc, asbestos), diet (consumption of animal fats, dairy products, soybeans), exercise, obesity.
The incidence of epithelial ovarian, fallopian or uterine tubes and peritoneal carcinoma cancer increase with age, the risk of EOC increases 2 % for each additional year of age in women <50 years and 11 % ≥50 years of age. The risk of ovarian cancer is higher in women with infertility and decreased in those taking oral contraceptives or multiparous. Early menarche (before age 12) is associated with increased risk of inconsistent form EOC. Older age at menopause (after age 52) is associated with increased risk of EOC and statistically increases the risk in women with menopause depues presentation of 52 years of age compared with those ≤45 years of age (relative risk [RR] 1.46, 95 % CI: 1.06–1.99) and is related to the increase in the total number of ovulations in a woman’s life, an increase of 2–7 % risk of EOC for each additional year of ovulation (RR 1.07, 95 % CI: 01.05–01.08) [38]. Nulliparous women are at greater risk of EOC and women who have had children the risk is lower (hazard ratio 0.49, 95 % CI 0.25–0.95) or history of term pregnancy [38, 39], a significantly lower risk nulliparous women compared with multiparous (RR 0.71, 95 % CI 0.27–0.30 % versus 0.59–0.87), found the risk of EOC decreased with increasing parity [30–32] and women with at least a full term pregnancy, the risk decreased 8 % on each additional pregnancy (95 % CI: 0.85–0.99), even multiparity also decreases the risk of cancer uterine tube or tubes, the history of multiple pregnancy or late pregnancy in women (>35 years) protects against EOC. The spontaneous or induced abortion is not associated with increased risk of EOC [40, 41].
Infertility is a risk factor for EOC, but the drugs used for induction of ovulation in infertility treatment do not increase the risk, but the risk of ovarian cancer in women attempting pregnancy increased over 5 years compared with those who tried less than a year (OR: 2.76, 95 % CI: 1.91–3.74) [7]. Endometriosis is associated only with some histological subtypes of EOC and the greatest risk is with clear cell (OR 3.05, 95 % CI 2.43–3.84), endometrioid (OR 2.04, 95 % CI: 1, 67–2.48), and low-grade serous (OR 2.11, 95 % CI 1.39–3.20), not relevant to the high-grade serous (OR 1.13, 95 % CI 0.97–1.32) or mucinous (OR 1.02, 95 % CI 0.69–1.50) [7, 9] and the risk of malignant transformation of ovarian endometriosis was 2.5 %. The EOC associated with endometriosis develops in women younger and has a better prognosis than most cases of EOC [4]; age women with clear cell EOC originated in an area of endometriosis are younger (49 versus to 59 years old) and better overall survival (196 vs 34 months) than women without endometriosis [11]. Women with PCOS are at increased risk of epithelial ovarian cancer (OR 2.52, 95 % CI 1.08–5.89). The absolute risk of ovarian cancer with postmenopausal hormone therapy is minimal or no statistically significant increase with estrogen-progestin combination compared to placebo (42 versus 27 per 100,000 person-years, HR 1.6, 95 % CI 0.8–3.2) and only risk is greater for estrogen alone compared with estrogen-progestin therapy. Postmenopausal hormone therapy is associated with increased risk of cancer of the uterine tube. The use of an intrauterine device and an increased risk of ovarian cancer (RR 1.76, 95 % CI 1.08–2.85) [2–4, 6, 7] and pelvic inflammatory disease is useful marker for cancer ovary [42].
46.4.1 Genetic Factors in Ovarian Cancer
Several susceptibility genes identified for the EOC, mainly BRCA1 and 2 and mismatch repair genes (associated with Lynch syndrome), others include genes RAD51C, RAD51D and BRIP1; these BRCA gene mutations and Lynch syndrome relations with the 10–15 % of EOC, also a personal or family history of breast cancer are risk factor for EOC, however, mutations in the BRCA genes account for most of the increased risk, women who are negative for BRCA mutations and having at least three relatives in the same lineage with breast not increase the incidence of EOC compared with the general population cancer, even women with breast or ovarian cancer who are BRCA1 mutation negative or relatives women have increased risk of breast cancer only a moderate risk of EOC statistically significant increase, but women with BRCA gene mutations are at increased risk of breast and EOC [5, 8]. The risk of EOC is 35–46 % for BRCA1 mutation carriers and 13–23 % in BRCA2 mutation carriers. Ovarian syndrome site specific cancer; presently considered part of breast cancer and EOC syndrome, in North America 13–15 % of women with EOC have germline mutation of BRCA mutations represent BRCA most hereditary ovarian cancers, women with BRCA1 gene mutations develop ovarian cancer at an earlier age than other women, with mean age at diagnosis of 50 years and an incidence of 2–3 % of EOC to 40 years old and the average age at diagnosis of EOC in BRCA2 mutation carriers is 60, similar to the general population, women with this mutation reached an incidence of 2–3 % at age 50 [5, 8]. The stage presentation of EOC is similar to the mutation of the BRCA gene and the general population, 70 % of patients present with stage III or IV and women carried the BRCA mutations are more likely to have EOC high grade than controls of the same age and carriers of BRCA1 and 2 genes have a similar histopathological types that the general population; serous adenocarcinoma is the most common histopathological phenotype and mucinous or borderline types are rare, the carriers of BRCA mutations, including BRCA2, have a better prognosis than non-carriers, better stage, grade, and lower mortality from all causes to 5 years adjusted for histology, BRCA1 gene carriers versus noncarriers: 45 front 47 %, HR 0.73, 95 % CI: 0.64–0.84; BRCA2 carriers versus noncarriers, 36 vs. 47 %, HR 0.49, 95 % CI 0.39–0.61, further improves survival due to the sensitivity for Qt based on platinum more of these tumors to sporadic cases, without pregnancy, improved for BRCA mutation carriers is short-term prognosis, but have higher rate survival in the BRCA group at 3 years (75 versus 65 %), with no difference at 5 years or more [1, 4, 12]. BRCA gene mutations are risk factors for uterine ncánceres tuba or tubes and peritoneal, the carriers of BRCA mutation rarely present with a tumor or recognized as primary cancer of uterine tubes (the lifetime incidence and 0.6 %). The BRCA mainly BRCA1 mutations identified in 16–43 % of women with primary fallopian tube cancer 5,8,12; pair genetic testing BRCA gene mutation is offered to women with these tumors, along with prophylactic surgery with bilateral salpingo-oophorectomy, reduces the risk of peritoneal cancer at 1.7 % (and range from 0.5 % to 10.7 %) in patients with BRCA mutations 20 years after prophylactic salpingo-oophorectomy cumulative incidence of carcinoma peritoneal was 4.3 %, which is greater for BRCA1 carriers without excluding an association with BRCA2, have been reported, peritoneal carcinomas in some women with BRCA2 mutations [38–42].
Lynch Syndrome or hereditary nonpolyposis colorectal cancer (HNPCC) is associated with other cancers primary, including cancers of the endometrium, vary, gastrointestinal and urogenital. Colorectal cancer is characteristic of Lynch syndrome, endometrial cancer is the second most common malignancy in women affected (70 %), but the frequency is increased to the EOC. EOC risk in women with Lynch syndrome is from 3 % to 14 % compared with 1.5 % of the general population, people with Lynch syndrome represent 1 % of the EOC and develop more young women than other women with EOC (43–50 years vs. 60 years), histopathology and survival are similar in women with Lynch syndrome compared to sporadic EOC, and most serous papillary histological type, also other subtypes, including endometriod cancer, mucinuos and clear cell, present in women with Lynch syndrome and usually the EOC this stage I or II with no difference in overall survival rate at 5 years [5, 8, 12]. Other genetic factors such as genes for FANCD1 Fanconi anemia, whilw mono-allelic mutations increase the risk of cancers associated with BRCA-1 mutations, this and other genes of Fanconi anemia, play a role in homologous recombination, some mutations of genes in this pathway are associated with an increased risk of breast cancer, including partnership between the BRCA genes, Fanconi anemia and EOC [5, 8, 9, 12].
Environmental factors, current or past smoking only increases the risk of mucinous EOC compared with never smokers (RR 2.1, 95 % CI 1.7–2.7), did not increase the risk of serous EOC (RR 1.0, 95 % CI 0.8–1.2) and mucinous EOC, the risk increased with increasing levels of cigarette consumption. The association of use in the genital or perineal talc increases (relative risk 1.33, 95 % CI 1.16–1.45) in population, but not in trials where no increase was found in the frequency EOC by the use of talc [5, 8, 9, 12]; talc is structurally similar to asbestos, a known carcinogen and exposure to asbestos increases the risk of EOC (standardized mortality ratio 1.77, 95 % CI 1.37–2.28), painting, welding, and other chemicals are associated with increased risk of cancer of the uterine tube [11, 12, 43].
There is increased risk of EOC in women with a high intake of animal fats, but not for the consumption of dairy products, only the daily increase of 10 g of lactose RR 1.13, 95 % CI 1.5–1.22), but is insufficient to establish dairy intake as a risk factor for the EOC, the high soy intake decreases the risk of EOC, nor supplementation is associated with vitamin D and ovarian cancer risk [5, 8, 9, 12].
Physical activity reduces the risk of EOC in women with high levels of activity others suggest increased risk with vigorous activity [44–47]. Obesity or increased body mass index (BMI) increases the risk of EOC, the association between obesity (BMI of 30 kg/m2 or more) and EOC, the OR 1.3, 95 % CI 1.1–1.5) also the risk of death from EOC is greater in women with higher BMI (35–40 kg/m2) compared with normal weight (BMI 18.5–24.9 kg/m2) (RR 1.51, 95 % CI 1.12–2.2). Other factors, there is no relationship between alcohol consumption and risk of EOC or use of anti-inflammatory drugs, including aspirin alone in the association of a history of pelvic inflammatory disease and EOC [44, 47].
The factors include reduced risk of EOC, oral contraceptives, multiparity, bilateral salpingo-oophorectomy, tubal uterine tubes, hysterectomy, breastfeeding. Prolonged use of oral contraceptives (OC) reduces the risk of EOC, comparing women who had never used OCs, with any use of AO is associated with reduced risk of EOC (RR 0.73, 95 % CI: 0.70–0.76) and the reduction is greater with prolonged use of OCs (RR less than 20 % for every 5 years of use, use for 15 years, reduced to 50 %) and the protective effect remained 30 years after stopping OCs, the effect is removed over time (for women with 5 years of OC use, reducing the risk of EOC in 10 years compared to 20–29 years after stopping OCs was 29 compared with 15 %), mainly for mucinous EOC even OC use is associated with lower risk of cancer of the uterine tuba [5, 8, 9, 11, 12, 48, 49]. The OC with the current standard or low (≤35 mcg of ethinyl estradiol) doses are associated with similar or lower risk of EOC compared with OC higher doses used previously, data on contraceptive use of estrogen and progestin oral not reported (ring, patch, etc.) for the prevention of EOC, the OC is used in the prevention of EOC for women at high risk for this disease; bilateral salpingo-oophorectomy (BSO) reduces the risk of EOC, but some women still develop carcinoma primary peritoneal. Prophylactic BSO surgery is to reduce the risk of ECO and cancer of the uterine tube or tubes (which are hidden and are reported in women undergoing BSO). The BSO in premenopausal or early onset of menopause is associated with adverse long-term effects but should discuss the risks and benefits to health. Oophorectomy or unilateral salpingo-oophorectomy and risk of ovarian cancer, increased risk of EOC, especially if hysterectomy is performed (odds ratio [OR] 4.2, 95 % CI 1.3–13.8), due to ovarian pathology which was the original indication for surgery. The removal of the uterine tubes or tubes at the time of hysterectomy or tubal uterine tubes is recommended. Hysterectomy without oophorectomy was associated with reduced risk of EOC (OR 0.66, 95 % CI 0.50–0.86). Women with a history of tubal uterine tubes reduces the risk of EOC (OR 0.69, 95 % CI: 0.64–0.75), too, reduced BRCA1 in 60 %, after adjustment OC use, parity, history of breast cancer and ethnicity [48, 49]. A history of OC use and ligation of uterine tubes is more protective (72 % reduced risk), because the tuba uterine neoplasia is a precursor lesion to EOC, there are no data on the impact of hysteroscopic sterilization methods on the risk EOC. Breastfeeding> breastfeeding 12 months compared with shorter breastfeeding reduces the risk of EOC (OR 0.72, 95 % CI 0.54–0.97).
46.5 Diagnosis
46.5.1 Clinical Findings
Attempts to develop programs for early detection of EOC tumors using markers and imaging studies have not yet been successful. The clinical picture of the EOC and cancers of the uterine tube or tubes and peritoneum are similar to common pathogenesis and start in the oviducts, the EOC is the term used to refer to the disease in any of these three sites, the EOC has been called the silent murderer because symptoms present late in the course of the disease, with abdominal distension, nausea, anorexia, early satiety due to the presence of ascites and metastasis in omentum or bowel loops; dyspnea occasionally occurs spill pleural, these symptoms occur in many women in early stages of the disease [50]. Most women with EOC have abdominal or pelvic symptoms before diagnosis, but 7 % are asymptomatic when studied in the consultation and 23 % when the records are reviewed, the difference is due to bias by the patient.
Women do not always seek help at the right time, or is offered a timely diagnosis. No recognition of the seriousness of symptoms (which may be the result of low awareness of symptoms) the patient appears to be mediated most important factor leading to a longer seeking help for symptoms of ovarian cancer. The nonspecific nature of the symptoms of ovarian cancer (e.g., swelling or sore lower back) can make it difficult to discern when a body change is serious, which could contribute to the lack of recognition of the seriousness of symptoms or attribution symptoms erroneous. Fear of cancer has also been found to increase the time to seek help, while no conclusive evidence of the effects of age 55. Women were still able to recognize only just over half of the symptoms (usually M = 6.3/10; subgroup M = 6.1/10) when prompted, suggesting that there is a need to improve awareness symptoms potentially indicative of EOC [51]. Routine pelvic examinations detect only one ovarian cancer in 10,000 asymptomatic women.
The symptoms were most commonly reported were pain, abdominal discomfort as inflammation and swelling that are confused with symptoms attributed to many conditions, but are most common and frequently repeated and more severe in women before the diagnosis of EOC, in women with pelvic masses before surgery 34.3 % are diagnosed with EOC, EOC women had higher rates compared to those with benign masses of the following clinical data: enlarged abdomen (64 versus 56 %), swelling (70 vs. 49 %) and urinary tract symptoms (especially emergency) (55 vs. 31 %). Pelvic pain, abdominal pain, abdominal mass, difficulty eating and constipation were more frequent in women with EOC patients during the consultation, but not in those with pelvic masses [50, 51] Fig. 46.2.
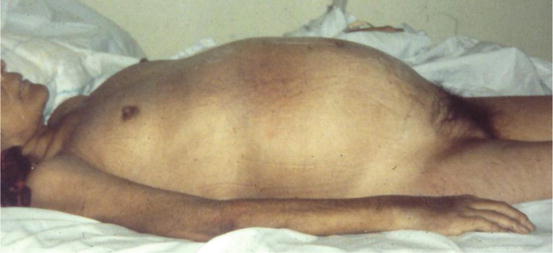

Fig. 46.2
Clinical appearance of women with ovarian cancer in later stages
The pattern and quality of symptoms in women with EOC are turning more frequently (20–30 times compared to 2–3 times a month.) Are more severe and of shorter duration (less than 3–6 months compared to a year or more) patients with EOC are more likely to have multiple symptoms (the triad of abdominal bloating, increased abdominal circumference and urinary urgency are present in 44 % versus 8 %; patients with EOC were more likely to go with symptoms such as abdominal bloating and gastrointestinal symptoms in the 6 months prior to cancer diagnosis. duration of the onset of symptoms at diagnosis of ovarian cancer varies 30 % of women had symptoms of 0–2 months 35 % during 3–6 months, 20 % for 7–12 months and 15 % over 12 months. Women who ignored their symptoms are more frequent in advanced stages. When symptoms potentially identify early stage recognized. Women with early stage EOC have symptoms, but less frequently than patients with late stage [50, 51], 89 versus 97 %. The primary objective of early detection limited to the ovary reduces mortality from EOC-rate 5-year survival of 80–90 %, unfortunately 80 % positive lymph nodes or distant metastases at diagnosis with survival rates 5 years that decrease 32–19 % for advanced disease, the secondary goal is the detection and treatment of advanced disease is to do as soon as possible. Cure rates with optimal <1 cm debulking of gross residual disease after surgery was achieved in 30–40 % compared to those optimal surgery is not achieved the cure rate is 15 versus 20 %. The most significant factor associated with optimal cytoreduction is the volume of disease at presentation [50, 51]. The evaluation of the symptoms of women with EOC identified more completely resectable disease. Women 50 years and older with symptoms associated with EOC were evaluated with CA-125 and transvaginal ultrasound for 7 months up, 16 % had an abnormal result and underwent further evaluation EOC 0.8 % were diagnosed cancers or uterine tube or peritoneal; women with EOC or uterine cancer or peritoneal tubas, 72 % complete cytoreduction was performed compared with 23 % in the control group of women with EOC that was presented during the study period [52].
Specific patterns of symptoms and EOC are identified, early detection of the disease is possible, but the issue is whether the diagnosis of EOC 3–6 months before improve prognosis, the potential impact of early detection depends on the amount time it takes to move from disease confined to the ovary or uterine tubes or for metastatic disease tubas and how quickly metastases increase in volume and there are no data relating to the doubling time for EOC, only the average time to develop metastases in the port site after laparoscopy in EOC was 17 days and it is estimated that ovarian tumors spend more than 4 years in stage I or II and 1 year in stage III or IV to be evident clinically, for recurrent EOC, the average time of CA -125 (for to assess tumor volume) doubles in 40 days, but the time interval for duplication is extensive and the prognosis is significantly worse in patients with rapid doubling compared with those with longer doubling [53, 54]. Symptoms associated with EOC are often nonspecific and no gynecological and patients or physicians do not consider the possibility of EOC when these symptoms, the clinical challenge is to identify which symptoms warrant further evaluation to EOC, without performing unnecessary tests [50, 55].
46.5.2 Symptoms of Suspicion
Risk assessment for EOC depends on the age and background I inherit-family, it is more effective to focus on the symptoms of women with increased risk (>40 years of age or family history of EOC or hereditary cancer syndromes). Women who have symptoms suggestive of EOC or detected during a routine are: bloating, urgency, urinary frequency, difficulty eating or feeling full, abdominal or pelvic pain, these women who complain of these symptoms should be evaluated with a detailed history to evaluate other possible symptoms of EOC and additional information about each symptom (e.g., frequency, severity) [50, 55].
The symptoms of new onset coexist with other symptoms occur daily and are more severe than expected with further evaluation, e.g., persistent bloating is associated with EOC [56] or even predicted the risk of EOC in 2 years on the basis of family history of EOC or symptoms (e.g., bloating or abdominal pain) [57], for a period of 2 years, 63 % of EOC diagnosed before 10 % in women at increased risk. The main predictors were: abdominal distension family history of EOC, postmenopausal bleeding and loss of appetite; has developed a symptom index to assist clinicians in the evaluation of women with early symptoms of EOC, the index is considered positive if a woman refers to any of the following symptoms; further discomfort for her in the last year and when occur more than 12 times a month pelvic or abdominal pain, increased abdominal size or bloating, difficulty eating or feeling full quickly, the track a positive index includes evaluating a woman with suspected EOC, with assessment of risk factors, images of the pelvis, serum CA- 125 and referral to a gynecologic oncologist, surgical exploration should not be made only on the basis to an index of positive symptoms pelvic pain or abdominal pain, increased abdominal size or bloating and difficulty eating or feeling full were the symptoms that are associated most significantly with EOC when present for at least 1 year and are presented more than 12 days per month, when this reference pattern is related to determining the performance index in stages of the disease, the sensitivity for early and late stage was 57–80 %, according to the age of the patient, sensitivity and specificity in women <50 years was 87 % and ≥50 years 67–90 %, comparing the usefulness of the index population average risk of the general population [50




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree