CHAPTER 2 Evaluation of the Symptomatic Patient: Diagnostic Breast Imaging
Screening mammography is used to detect breast cancer in asymptomatic women. In order to be cost-effective and efficient, screening mammography is performed by a technologist and batch-read at a later time by the radiologist. Most screening mammography findings for which patients are called back prove to be benign. The average callback rate for screening mammography is about 8% to 10%.1 Of these callbacks, only about 15% will prove to be suspicious after diagnostic workup and require biopsy. Of the biopsies, about one third (30% to 35%) will yield a diagnosis of cancer. Screening mammography is intended for detection, not analysis, of potential abnormalities. Diagnostic evaluations involve the use of additional mammographic views, such as spot compression and magnification, and other breast evaluation techniques, such as ultrasound, physical exam, and ductography, to analyze potential abnormalities identified on screening mammography and to evaluate symptomatic patients. A diagnostic study is directed and supervised by a radiologist, and patients are given their results at the conclusion of the examination. Questions to be addressed through workup of a screening mammography finding are:
Magnification views are utilized to evaluate morphologic features, such as the margins of masses and the shapes of microcalcifications. The Breast Imaging Reporting and Data System (BI-RADS) lexicon is a classification scheme used to help standardize the description and disposition of breast lesions seen on mammography, ultrasound, and MRI.2 BI-RADS categories 1 and 2 are used to describe a negative study and a study in which there are benign findings, respectively. Category 3 is used to describe probably benign findings, with a less than 2% chance of malignancy, which can be followed in 6 months.3 Patients with new or enlarging solid masses or increasing clustered microcalcifications that are not classically benign require biopsy. Category 4 is used to describe suspicious findings (greater than 2% chance of malignancy) that require biopsy. BI-RADS category 5 lesions are highly suspicious findings, having a 95% or higher likelihood of malignancy. Category 6 is used in patients with a known breast cancer who are undergoing neoadjuvant chemotherapy or additional imaging studies (Table 1).
Another primary role of diagnostic workups is to evaluate symptomatic patients. Commonly evaluated complaints include palpable lumps, an area of thickening, pain, and nipple discharge. The use of ultrasound in combination with mammography is extremely important in the symptomatic patient because some breast cancers may not be detected mammographically. Real-time evaluation by the radiologist is often necessary to detect subtle cancers. A report by the Physicians Insurers Association of America (PIAA) noted that a large percentage of suits involve women presenting with clinical symptoms and that a common reason for litigation was the claim that the physical symptoms “failed to impress” the physician.4 The negative predictive value of a negative mammography and ultrasound is estimated to be 95% to 99%.5–8 However, despite this high negative predictive value, a biopsy may still be warranted in the setting of a suspicious clinical finding.
MAGNETIC RESONANCE IMAGING (MRI) OF THE BREAST
Dynamic contrast-enhanced (DCE) MRI of the breast has been shown to be extremely sensitive in the detection of invasive breast cancer and is not limited by breast tissue density. Current indications include high-risk screening, evaluation for an unknown primary carcinoma, preoperative evaluation in patients with known breast cancer, evaluating response to neoadjuvant therapy, and suspected recurrence. However, the utility of DCE MRI has been limited by its variable sensitivity for ductal carcinoma in situ (DCIS). In general, the role of DCE MRI as a problem-solving tool in the evaluation of suspicious imaging or clinical findings is unclear. A negative MRI should not be used to avoid biopsy of suspicious findings on mammography or ultrasound or of a suspicious clinical finding.
NEEDLE BIOPSY PROCEDURES
Lesions categorized as suspicious following diagnostic workup require biopsy. Image-guided percutaneous needle biopsy techniques are firmly established as a valid replacement for surgical excisional biopsy, both in the evaluation of suspicious imaging findings and in diagnosing breast cancer.9–19 Multiple studies have demonstrated the accuracy of percutaneous large-core breast biopsy for nonpalpable lesions to be comparable to that of needle localization and open surgical biopsy. Percutaneous needle biopsy is less expensive and less invasive than surgical biopsy and does not deform or significantly scar the breast. Its use has decreased the number of unnecessary surgical biopsies for benign lesions and reduces the number of surgeries required to treat patients with cancer. Instead of two surgical procedures to diagnose and treat breast cancer (excisional biopsy for diagnosis, followed later by a separate sentinel node biopsy or axillary dissection), a single surgical procedure can often be performed. The average number of surgeries performed in women with cancers diagnosed preoperatively using percutaneous needle biopsy is significantly lower than in women whose cancer was surgically diagnosed.20–24 In addition, a preoperative tissue diagnosis allows patients to have a more informed and thorough discussion of cancer treatment options before surgery.
Ultrasound is the guidance method of choice for percutaneous interventional procedures of nonpalpable breast lesions. In addition, ultrasound guidance may be useful for selected palpable masses that are small, mobile, or vaguely palpable, in which palpation-guided biopsy may prove difficult.25,26 Ultrasound as a guidance modality for cyst aspiration, needle localization, and biopsy procedures is preferred for several reasons. These include patient tolerance, accuracy, speed, real-time visualization, accessibility to all areas of the breast and axilla, relatively low cost, and lack of ionizing radiation. Most ultrasound-guided biopsy procedures are performed for masses. Even when seen on ultrasound, microcalcifications are usually best biopsied stereotactically, unless an associated mass is identified on ultrasound.
Cyst Aspiration
Benign (simple) cysts can be accurately characterized using high-resolution ultrasound imaging and do not require aspiration or biopsy.27,28 Incidental complicated cysts (circumscribed round or oval masses with posterior enhancement, homogeneous low-level internal echoes, or mobile debris) can be safely followed.29–32 Aspiration is reasonable for isolated complicated cysts that are enlarging or newly palpable. In addition, simple cysts may be aspirated in patients desiring symptomatic relief. For incidental nonpalpable lesions that cannot be reliably classified as simple or complicated cysts, and would require biopsy if solid, ultrasound-guided cyst aspiration is indicated for differentiation.
Routinely sending aspirated cyst fluid to cytology can lead to unnecessary surgical biopsies because of false-positive fluid analysis. Unless the fluid is unusually bloody, it can be discarded.33–35 Bloody cyst fluid, although most likely benign, has been associated with intracystic papillomas. In cases in which bloody fluid is obtained upon aspiration and the cyst does not completely resolve, core needle biopsy of the residual cyst may yield a more definitive diagnosis than fluid analysis, possibly averting unnecessary surgical biopsy.
Fine-Needle Aspiration Biopsy
High-quality, fine-needle aspiration breast biopsies (FNABs) require both a skilled cytopathologist and on-site evaluation to establish adequacy of sampling. Although FNAB can provide quicker results and reduce patient anxiety because of faster processing time, accuracy should not be compromised for the sake of speed. Several studies, including the multicenter randomized trial performed by the Radiation Oncology Diagnosis Group V (RDOGV), have shown large-core needle biopsy to be superior to FNAB.36–38 In addition, unlike core needle biopsy, FNAB cannot reliably distinguish in situ from invasive carcinoma. Patients whose cancer has been diagnosed as invasive preoperatively can undergo a lumpectomy and lymph node dissection in a single surgical procedure, as opposed to two separate surgeries when diagnosed using FNAB. Given its superior accuracy, diagnostic utility, and safety, with few significant complications, core needle biopsy is preferred over FNAB.
14-Gauge Core Needle Biopsy
Image-guided percutaneous needle biopsy is a less invasive and less expensive alternative to surgical excisional biopsy in the diagnosis of breast cancer. Fourteen-gauge needles have been found to be more accurate than smaller-diameter core biopsy needles.39 To ensure accurate sampling, it is important to obtain an adequate number of samples and to be certain that the biopsy needle traverses the mass.
Vacuum-Assisted Core Biopsy
Vacuum-assisted core biopsy devices allow the acquisition of larger amounts of tissue per core biopsy specimen. Compared to 14-gauge spring-loaded biopsies, which on average yield 18 mg of tissue per core specimen, the 11-gauge vacuum-assisted devices can obtain core samples averaging 95 mg.40 Complication rates for 11-gauge vacuum-assisted biopsy have not been shown to be significantly different than those for 14-gauge automated core needle biopsy.41,42
Larger tissue volume devices have facilitated the sampling of microcalcifications during stereotactic biopsy. In addition, the frequency of histologic underestimation, imaging-histology discordance, and rebiopsy is lower for 11-gauge vacuum-assisted stereotactic biopsy than for 14-gauge core needle biopsy.43–48 However, because these types of sampling errors occur in only a small percentage of patients who undergo 14-gauge ultrasound-guided biopsy, improvements in these rates may benefit relatively few patients. Philpotts and colleagues42 reported no significant differences in the outcomes of sonographically guided core biopsies performed with the automated gun compared with those performed with a vacuum-assisted device, in terms of missed cancers, underestimation, or the need (immediate or delayed) for a second biopsy.
If enough samples are taken, vacuum core biopsy devices can remove a large portion of a lesion. However, they are only approved for diagnostic purposes and not for excision or removal. Although there is little data available, removal of most of a lesion through vacuum core biopsy has been suggested for possible relief of painful, symptomatic fibroadenomas. Removing all imaging evidence of a lesion does not equate to complete excision of the pathologic abnormality. Liberman and colleagues49 reported that for cancers in which the imaging findings had been removed by vacuum-assisted biopsy, 79% had residual cancer at excision. The use of large-volume vacuum core biopsy, instead of open surgical excision, to evaluate high-risk lesions found at 14-gauge biopsy (papillomas, radial scars, atypia, or suspected phyllodes tumors) has not been extensively studied. Further investigation is needed to assess the potential benefits of completely removing the imaging findings of lesions versus sampling them.
Imaging-Histology Correlation and Follow-up
The false-negative rate for ultrasound-guided core-needle biopsy procedures is between 0% and 1.26%.50 It is important to remember that needle biopsy of breast lesions is a sampling procedure and that undersampling can occur. Low falsenegative rates and high accuracy are only attained by combining precise targeting and sufficient sampling with systematic radiologic and pathologic correlation.
In order for needle biopsy procedures to attain a level of accuracy similar to that of needle localization and surgical excisional biopsy, pathology results must be correlated with the imaging findings.18,51–53 A discordant finding, one in which the histopathologic findings do not provide a sufficient explanation for suspicious imaging findings, warrants a repeat biopsy. This is usually in the form of a surgical biopsy, owing to the possibility of a second discordant result on repeat needle biopsy. Imaging-histologic discordance rates for 14-gauge ultrasound-guided needle biopsy have been reported as high as 7.7%. Crystal and associates18 found that 12 of 323 cancers were missed at initial ultrasound-guided 14-gauge needle biopsy. Of these missed cancers, 7 were found immediately at rebiopsy prompted by discordant results, 2 at immediate rebiopsy because of indeterminate pathology findings, and 3 at later follow-up. Sauer and coworkers,51 in their study of 962 lesions that underwent 14-gauge core biopsy under three-dimensional ultrasound guidance, reported a 3% discordance rate, of which 27.6% proved to be malignant on rebiopsy. Additionally, Liberman and colleagues52 reported a 3.3% discordance rate out of 580 lesions biopsied under ultrasound guidance. At rebiopsy, 10.5% of these discordant lesions proved to be carcinoma. Therefore, careful imaginghistologic correlation will allow the detection of a significant number of false-negative results immediately after needle biopsy, thereby avoiding delays in diagnosis.
HIGH-RISK LESIONS FOUND AT CORE NEEDLE BIOPSY
Lobular Neoplasia and Atypical Ductal Hyperplasia
Underestimation of disease occurs when a lesion diagnosed as benign or atypical by core needle biopsy is upgraded to cancer upon surgical excision. Although the upgrade rate of atypical ductal hyperplasia (ADH) at needle biopsy to DCIS at surgical excision has decreased because of larger tissue volumes obtained with 11-gauge vacuum-assisted technique, upgrade rates as high as 21% have been reported.54,55 Even when 100% of the mammographic lesion was removed at stereotactic biopsy, 8% of cases with ADH were still upgraded from ADH to DCIS at excision.54 Therefore, a diagnosis of ADH at core needle biopsy warrants surgical excision.
The diagnosis of lobular neoplasia (lobular carcinoma in situ [LCIS] and atypical lobular hyperplasia) confers an increased risk for developing infiltrating carcinoma (ductal or lobular) in either breast. However, recommendations for surgical excision following a diagnosis of lobular neoplasia on core needle biopsy have not been standardized. Recent studies have suggested that, similar to ADH, surgical excision may be warranted when a diagnosis of lobular neoplasia is obtained at core needle biopsy.56–58 However, as more data is accumulated, the decision to excise may be further refined based on the variety of LCIS and the relationship of the pathology to the radiographic findings that prompted biopsy.
Radial Scars and Papillary Lesions
Radial scars, also termed complex sclerosing lesions when larger than 1 cm, are not infrequently associated with DCIS, tubular carcinoma, ADH, and LCIS.59–61 Therefore, surgical excision is recommended when a diagnosis of radial scar is obtained at core needle biopsy. Although further data are needed, recent studies have suggested that in selected subsets of patients, incidental radial scars found at core needle biopsy can potentially be safely followed rather than excised.62–65
A diagnosis of benign papilloma found at core needle biopsy of a radiographically suspicious mass should be considered discordant and calls for surgical excision. In addition, atypical papillary lesions should be excised.66,67 For benign papillary lesions that are incidental or concordant with the imaging findings, recommendations for imaging follow-up versus excision have not been standardized. A recent study and literature review reports upgrade rates for benign papillomas diagnosed by core biopsy of 5% and 8%.68 However, upgrade rates of benign papillomas diagnosed by large-gauge vacuum core biopsy may be lower.
Cellular Fibroadenomas and Phyllodes Tumors
Phyllodes tumors are relatively uncommon, accounting for less than 1% of all breast neoplasms.69 Although they are most often benign, they can be locally aggressive. An actively growing cellular fibroadenoma may be difficult for the pathologist to distinguish from a phyllodes tumor when sampled by core biopsy. Therefore, surgical excision should be considered when a diagnosis of cellular fibroadenoma is obtained at core biopsy of a mass that is rapidly growing, clinically discordant, or has unusual imaging features.70
1 Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology. 2006;241:55-66.
2 American College of Radiology (ACR). Breast Imaging Reporting and Data System Atlas (BI-RADS® Atlas). Reston, Va: American College of Radiology, 2003.
3 Sickles EA. Periodic mammographic follow-up of probably benign lesions: results in 3,184 consecutive cases. Radiology. 1991;179(2):463-468.
4 Report from the Physician Insurers Association of America (PIAA), 2005.
5 Dennis MA, Parker SR, Klaus AJ, et al. Breast biopsy avoidance: the value of normal mammograms and normal sonograms in the setting of a palpable lump. Radiology. 2001;219:186-191.
6 Soo MS, Rosen EL, Baker JA, et al. Negative predictive value of sonography with mammography in patients with palpable breast lesions. AJR Am J Roentgenol. 2001;177:1167-1170.
7 Moy L, Slanetz PJ, Moore R, et al. Specificity of mammography and US in the evaluation of a palpable abnormality: retrospective review. Radiology. 2002;225:176-181.
8 Houssami N, Irwig L, Simpson JM, et al. Sydney breast imaging accuracy study: comparative sensitivity and specificity of mammography and sonography in young women with symptoms. AJR Am J Roentgenol. 2003;180:935-940.
9 Liberman L, Kaplan JB. Percutaneous core biopsy of nonpalpable breast lesions: utility and impact on cost of diagnosis. Breast Dis. 2001;13:49-57.
10 Mainiero MB, Gareen IF, Bird CE, et al. Preferential use of sonographically guided biopsy to minimize patient discomfort and procedure time in a percutaneous image-guided breast biopsy program. J Ultrasound Med. 2002;21:1221-1226.
11 Rubin E, Mennemeyer ST, Desmond RA, et al. Reducing the cost of diagnosis of breast carcinoma: impact of ultrasound and imaging-guided biopsies on a clinical breast practice. Cancer. 2001;91(2):324-332.
12 Parker SH, Jobe WE, Dennis MA, et al. US-guided automated large-core breast biopsy. Radiology. 1993:187.
13 Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology. 1994:193.
14 Crowe JPJr, Rim A, Patrick RJ, et al. Does core needle breast biopsy accurately reflect breast pathology? Surgery. 2003;134:526-528.
15 Meloni GB, Dessole S, Becchere MP, et al. Ultrasound-guided Mammotome vacuum biopsy for the diagnosis of impalpable breast lesions. Ultrasound Obstet Gynecol. 2001:18.
16 Simon JR, Kalbhen CL, Cooper RA, Flisak ME. Accuracy and complication rates of US-guided vacuum-assisted core breast biopsy: initial results. Radiology. 2000:215.
17 Buchberger W, Niehoff A, Obrist P, et al. Sonographically guided core needle biopsy of the breast: technique, accuracy and indications. Radiology. 2002:42.
18 Crystal P, Koretz M, Shcharynsky S, et al. Accuracy of sonographically guided 14-gauge core-needle biopsy: results of 715 consecutive breast biopsies with at least two-year follow-up of benign lesions. J Clin Ultrasound. 2005:33.
19 Smith DN, Rosenfield Darling ML, Meyer JE, et al. The utility of ultrasonographically guided large-core needle biopsy: results from 500 consecutive breast biopsies. J Ultrasound Med. 2001:20.
20 Smith DN, Christian R, Meyer JE. Large-core needle biopsy of nonpalpable breast cancers. The impact on subsequent surgical excisions. Arch Surg. 1997;132:260.
21 Kaufman CS, Delbecq R, Jacobson L. Excising the reexcision: stereotactic core-needle biopsy decreases need for reexcision of breast cancer. World J Surg. 1998;22:1028.
22 Liberman L, LaTrenta LR, Dershaw DD, et al. Impact of core biopsy on the surgical management of impalpable breast cancer. AJR Am J Roentgenol. 1997:168.
23 Lind DS, Minter R, Steinbach B, et al. Stereotactic core biopsy reduces the reexcision rate and the cost of mammographically detected cancer. J Surg Res. 1998;78(1):23-26.
24 Yim JH, Barton P, Weber B, et al. Mammographically detected breast cancer. Benefits of stereotactic core versus wire localization biopsy. Ann Surg. 1996;223:697-700.
25 Liberman L, Ernberg LA, Heerdt A, et al. Palpable breast masses: is there a role for percutaneous imaging-guided core biopsy? AJR Am J Roentgenol. 2000:175.
26 Lorenzen J, Welger J, Lisboa BW, et al. Percutaneous core-needle biopsy of palpable breast tumors. Do we need ultrasound guidance? Rofo. 2002:174.
27 Hilton SV, Leopold GR, Olson LK, Willson SA. Real-time breast sonography: application in 300 consecutive patients. AJR Am J Roentgenol. 1986:147.
28 Vargas HI, Vargas MP, Gonzalez KD, et al. Outcomes of sonography-based management of breast cysts. Am J Surg. 2004:188.
29 Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003:227.
30 Buchberger W, DeKoekkoek-Doll P, Springer P, et al. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol. 1999:173.
31 Mendelson EB, Berg WA, Merritt CR. Toward a standardized breast ultrasound lexicon, BI-RADS: ultrasound. Semin Roentgenol. 2001:36.
32 Venta LA, Kim JP, Pelloski CE, Morrow M. Management of complex breast cysts. AJR Am J Roentgenol. 1999:173.
33 Ciatto S, Cariaggi P, Bulgaresi P. The value of routine cytologic examination of breast cyst fluids. Acta Cytol. 1987:31.
34 Hindle WH, Arias RD, Florentine B, Whang J. Lack of utility in clinical practice of cytologic examination of nonbloody cyst fluid from palpable breast cysts. Am J Obstet Gynecol. 2000:182.
35 Smith DN, Kaelin CM, Korbin CD, et al. Impalpable breast cysts: utility of cytologic examination of fluid obtained with radiologically guided aspiration. Radiology. 1997:204.
36 Pisano ED, Fajardo LL, Caudry DJ, et al. Fine-needle aspiration biopsy of nonpalpable breast lesions in a multicenter clinical trial: results from the radiologic diagnostic oncology group V. Radiology. 2001:219.
37 Symmans WF, Cangiarella JF, Gottlieb S, et al. What is the role of cytopathologists in stereotaxic needle biopsy diagnosis of nonpalpable mammographic abnormalities? Diagn Cytopathol. 2001:24.
38 Clarke D, Sudhakaran N, Gateley CA. Replace fine needle aspiration cytology with automated core biopsy in the triple assessment of breast cancer. Ann R Coll Surg Engl. 2001:83.
39 Nath ME, Robinson TM, Tobon H, et al. Automated large-core needle biopsy of surgically removed breast lesions: comparison of samples obtained with 14-, 16-, and 18-gauge needles. Radiology. 1995:197.
40 Berg WA, Krebs TL, Campassi C, et al. Evaluation of 14- and 11-gauge directional, vacuum-assisted biopsy probes and 14-gauge biopsy guns in a breast parenchymal model. Radiology. 1997;205(1):203-208.
41 Kettritz U, Rotter K, Schreer I, et al. Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer. 2004:100.
42 Philpotts LE, Hooley RJ, Lee CH. Comparison of automated versus vacuum-assisted biopsy methods for sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2003:180.
43 Kettritz U, Rotter K, Schreer I, et al. Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer. 2004:100.
44 Darling ML, Smith DN, Lester SC, et al. Atypical ductal hyperplasia and ductal carcinoma in situ as revealed by large-core needle breast biopsy: results of surgical excision. AJR Am J Roentgenol. 2000:175.
45 Jackman RJ, Burbank F, Parker SH, et al. Atypical ductal hyperplasia diagnosed at stereotactic breast biopsy: improved reliability with 14-gauge, directional, vacuum-assisted biopsy. Radiology. 1997:204.
46 Jackman RJ, Nowels KW, Rodriguez-Soto J, et al. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999:210.
47 Philpotts LE, Lee CH, Horvath LJ, et al. Underestimation of breast cancer with 11-gauge vacuum suction biopsy. AJR Am J Roentgenol. 2000:175.
48 Philpotts LE, Shaheen NA, Carter D, et al. Comparison of rebiopsy rates after stereotactic core needle biopsy of the breast with 11-gauge vacuum suction probe versus 14-gauge needle and automatic gun. AJR Am J Roentgenol. 1999:172.
49 Liberman L, Kaplan JB, Morris EA, et al. To excise or to sample the mammographic target: what is the goal of stereotactic 11-gauge vacuum-assisted breast biopsy? AJR Am J Roentgenol. 2002:179.
50 Memarsadeghi M, Pfarl G, Riedl C, et al. Value of 14-gauge ultrasound-guided large-core needle biopsy of breast lesions: own results in comparison with the literature. Rofo. 2003:175.
51 Sauer G, Deissler H, Strunz K, et al. Ultrasound-guided large-core needle biopsies of breast lesions: analysis of 962 cases to determine the number of samples for reliable tumour classification. Br J Cancer. 2005:92.
52 Liberman L, Drotman M, Morris EA, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000:89.
53 Lee CH, Philpotts LE, Horvath LJ, Tocino I. Follow-up of breast lesions diagnosed as benign with stereotactic core-needle biopsy: frequency of mammographic change and false-negative rate. Radiology. 1999:212.
54 Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224(2):548-554.
55 Winchester DJ, Bernstein JR, Jeske JM, et al. Upstaging of atypical ductal hyperplasia after vacuum-assisted 11-gauge stereotactic core needle biopsy. Arch Surg. 2003;138:622-623.
56 Lechner MC, Jackman RJ, Brem RF, et al. Lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous biopsy with surgical correlation: a multi-institutional study [abstract]. Radiology. 1999;213(P):106.
57 Arpino G, Allred DC, Mohsin SK, et al. Lobular neoplasia on core-needle biopsy: clinical significance. Cancer. 2004:101.
58 Foster MC, Helvie MA, Gregory NE, et al. Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary? Radiology. 2004:231.
59 Sloane JP, Mayers MM. Carcinoma and atypical hyperplasia in radial scars and complex sclerosing lesions: importance of lesion size and patient age. Histopathology. 1993;23:225-231.
60 Frouge C, Tristant H, Guinebretiere J-M, et al. Mammographic lesions suggestive of radial scars: microscopic findings in 40 cases. Radiology. 1995;195:623-625.
61 Hassell P, Klein-Parker H, Worth A, Poon P. Radial sclerosing lesions of the breast: mammographic and pathologic correlation. Can Assoc Radiol J. 1999;50:370-375.
62 Philpotts LE, Shaheen NA, Jain KS, et al. Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology. 2000;216:831-837.
63 Kirwan SE, Denton ERE, Nash RM, et al. Multiple 14G stereotactic core biopsies in the diagnosis of mammographically detected stellate lesions of the breast. Clin Radiol. 2000;55(10):763-766.
64 Brenner RJ, Jackman RJ, Parker SH, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol. 2002:179.
65 Cawson JN, Malara F, Kavanagh A, et al. Fourteen-gauge needle core biopsy of mammographically evident radial scars. Is excision necessary? Cancer. 2003;97:345-351.
66 Leung J, Margolin FR, Lester SC, et al. Benign papillary breast lesions diagnosed at large-core needle biopsy: correlation with surgical pathology and clinical outcome [abstract]. AJR Am J Roentgenol. 2002;178:59-60.
67 Agoff SN, Lawton TJ. Papillary lesions of the breast with and without atypical ductal hyperplasia: can we accurately predict benign behavior from core needle biopsy? Am J Clin Pathol. 2004:122.
68 Mercado CL, Hamele-Bena D, Oken SM, et al. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology. 2006;238(3):801-808.
69 Liberman L, Bonaccio E, Hamele-Bena D, et al. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996:198.
70 Meyer JE, Smith DN, Lester SC, et al. Large-needle core biopsy: nonmalignant breast abnormalities evaluated with surgical excision or repeat core biopsy. Radiology. 1998:206.
CASE 1 Palpable axillary IDC, presenting as a growing mammographic mass simulating a lymph node
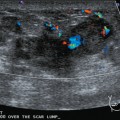
An 82-year-old woman identified a palpable right axillary tail mass. Mammography showed interval growth of a small mass (Figure 1), which had been evaluated 9 months before and was previously thought to be a lymph node (Figure 2). Ultrasound showed a corresponding 1-cm solid mass with lobular margins, with vascularity and no well-defined capsule (Figure 3). A surgical excisional biopsy revealed a 1.3-cm infiltrating ductal carcinoma (IDC) with ductal carcinoma in situ, most of which was within the invasive component. Carcinoma was transected at the anterior margin. The patient elected to undergo simple mastectomy to clear the margins, rather than undergo re-excision and radiation. No residual tumor was identified in the mastectomy specimen. An axillary sentinel lymph node was negative. The final stage was stage I, T1N0, estrogen receptor positive, HER2/neu negative.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree