Hirohiko Tsujii, Tadashi Kamada, Toshiyuki Shirai, Koji Noda, Hiroshi Tsuji and Kumiko Karasawa (eds.)Carbon-Ion Radiotherapy2014Principles, Practices, and Treatment Planning10.1007/978-4-431-54457-9_14
© Springer Japan 2014
14. Evaluation of Treatment Outcomes Using the Heavy-Ion Medical Accelerator in Chiba (HIMAC) Approach
(1)
Research Center for Charged Particle Therapy, National Institute of Radiological Sciences, Anagawa 4-9-1, Inage-Ku, Chiba 263-8555, Japan
Abstract
At present, most of the patients receiving carbon ion radiotherapy (C-ion RT) at the NIRS visit the clinic seeking this specific modality, and it is difficult to obtain consent for a randomized controlled study from these patients, so it may be unnecessary to conduct a phase III trial. However, in selected tumors where a high LET benefit could be appreciated, randomized studies should be performed. In addition, studies aimed at clarifying the usefulness of carbon ion radiotherapy and elucidating any advantages from hypo-fractionation should be considered. A good quality observational study, such as a multi-institutional prospective nonrandomized concurrent phase II clinical trial, is one such new approach, and it will be proposed not only to the Japanese but also to the international community in the fields of particle therapy and radiation oncology.
Keywords
Phase II clinical trialRandomized clinical trials (RCTs)14.1 Introduction
The pioneering work in carbon ion radiotherapy (C-ion RT) by Japanese and European investigators has generated great enthusiasm. However, as a particle therapy center is certain to be an extremely complex and expensive medical facility, it may become a source of disagreement in the radiation oncology community.
The paradigm of drug development in medical oncology from phase I to phase II to phase III trials remains unaltered, but this is not the case in radiation oncology.
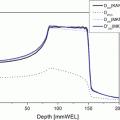
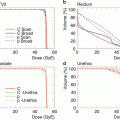
According to the presentations at the NCI “Workshop on Advanced Technologies in Radiation Oncology” in December 2006, there were only a few level I trials of new technologies in radiation oncology (http://www3.cancer.gov/rrp/workshop/2006AdvancedRadiationTech/presentations.html). For protons, the physical proof of better depth dose characteristics, virtually identical biological effects compared with X-rays, and the fact that decreased doses to normal tissues always result in decreased toxicity argue against conducting phase III trials comparing protons with X-rays [1].
Radiation oncology has not, until this decade, seen dramatic changes in technological innovations, and these give rise to new and complicated issues. For example, what kind of studies should be carried out to sort out the indications, advantages, and disadvantages of new techniques? In this chapter, the issue of evaluating the clinical results of carbon ion radiotherapy and their comparison with other modalities will be discussed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree