Extrapyramidal syndromes (ES) belong to the most common neurologic illnesses. Because new and promising therapeutic options are currently under development, there is a substantial demand for molecular imaging procedures with the potential to identify the pathologic changes of those illnesses. This article gives an overview of the current positron emission tomography and single photon emission computed tomography applications for diagnosing ES and focuses on their use in clinical practice.
Extrapyramidal syndromes (ES) belong to the most common neurologic illnesses. Because new and promising therapeutic options are currently under development, there is a substantial demand for molecular imaging procedures with the potential to identify the pathologic changes of those illnesses. This article gives an overview of the current positron emission tomography (PET) and single photon emission computed tomography (SPECT) applications for diagnosing ES and focuses on their use in clinical practice.
Among ES, the parkinsonian syndromes (PS) play a predominant role. This syndromatic umbrella term comprises 3 etiologically different entities: Parkinson disease (PD; idiopathic parkinsonian syndrome), atypical parkinsonian syndromes (aPS; parkinsonian syndromes caused by other neurodegenerative diseases), and symptomatic (secondary) parkinsonism (sPS), including some other major differential diagnoses. The group of aPS includes multiple system atrophies (MSAs, parkinsonian and cerebellar type), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and spinocerebellar ataxias (SCAs). Dementia with Lewy bodies (DLB), presumably reflecting a different variant of PD, also plays an increasing role. The sPS group includes vascular-, drug-, toxic-, metabolic-, and inflammatory-induced parkinsonism, normal pressure hydrocephalus, and, more rarely, cases of injury- or tumor-induced parkinsonism. Clinically, ET is the most important differential diagnosis in this group, when compared with PD and aPS.
The diagnosis of PS is usually established by using well-defined clinical criteria. Particularly in the early stages of disease, if clinical findings are subtle, mono-symptomatic (eg, isolated tremor), or equivocal, it may be difficult to establish the correct diagnosis. In the past various publications have addressed the issue of misdiagnosis from different points of view.
In patients suffering from PS, establishing an early and accurate diagnosis has an impact on their management, helps to avoid wrong treatment decisions, and may aid in selecting patients for therapeutic trials with newly developed drugs. Clinically, PS are characterized by disturbances in motor function, such as tremor, rigidity, bradykinesia, and postural abnormalities. Conventional structural imaging with CT and MRI has limited value in the early diagnosis of PS, because, in many instances, structural abnormalities may only be present in more advanced disease or may be difficult to assess in individual subjects. Conversely, molecular imaging with PET and SPECT offers various tools for the classification and differential diagnosis of PS. Apart from the assessment of brain perfusion, glucose metabolism, and cardiac sympathetic denervation, nuclear medicine techniques allow the imaging of key functions of neurotransmission involved in the etiology of neurodegenerative PS. Here, the dopaminergic system plays a dominant role in clinical routine assessment.
Imaging techniques
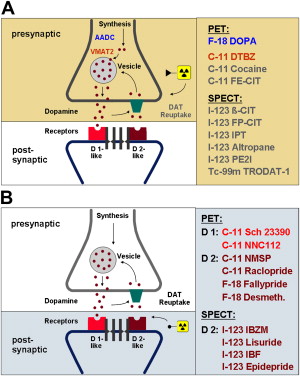
Since pathology in neurodegenerative PS involves the dopaminergic pathway, PET and SPECT investigations of the pathway deliver important diagnostic contributions. Presynaptic nigrostriatal terminal function can be assessed with radioligands suitable for imaging at least 3 different functions: the aromatic amino acid decarboxylase activity (PET: fluorodopa) ; the vesicular monoamine transporter type 2 (VMAT2, PET: dihydrotetrabenazine), and the plasma membrane dopamine transporter (DAT, PET and SPECT: cocaine analogs). Imaging of postsynaptic dopamine receptors has focused on the D 2 -like receptor system (PET: raclopride, desmethoxyfallypride[DMFP]; SPECT: iodobenzamide, iodobenzofuran, epidepride). The dopaminergic synapse and dopaminergic neurotransmission are schematically illustrated in Fig. 1 , highlighting the more widely used PET and SPECT tracers targeting these systems. Apart from the dopaminergic system, other approaches like assessment of brain glucose metabolism or cardiac sympathetic denervation of the heart (PET: hydroxyephedrine [HED], SPECT: metaiodobenzylguanidine [MIBG]) may also aid in the further classification of PS.
Characteristic PET and SPECT findings in PS
Clinicians seek answers to the following key questions: (1) whether the patient suffers from neurodegenerative PS or from a different (non-neurodegenerative) disease; (2) if neurodegenerative PS is likely, whether PD or aPS is the most probable diagnosis; and (3) if the latter is diagnosed, the aPS category.
The following sections briefly summarize major findings reported for the most frequent PS and provide a helpful scheme for answering the questions raised by the use of nuclear imaging techniques.
Characteristic PET and SPECT findings in PS
Clinicians seek answers to the following key questions: (1) whether the patient suffers from neurodegenerative PS or from a different (non-neurodegenerative) disease; (2) if neurodegenerative PS is likely, whether PD or aPS is the most probable diagnosis; and (3) if the latter is diagnosed, the aPS category.
The following sections briefly summarize major findings reported for the most frequent PS and provide a helpful scheme for answering the questions raised by the use of nuclear imaging techniques.
Neurodegenerative parkinsonian syndromes
PD
The predominant pathology in PD, which accounts for about 70% to 80% of PS, is the loss of dopaminergic neurons that project from the substantia nigra pars compacta in the midbrain to the striatum (putamen and caudate nucleus). The loss of neurons results in a dopaminergic deficit, which is believed to contribute to the clinical symptoms. Typically, the projections to the posterior putamen are affected earlier and to a greater extent than those to the caudate. The clinical diagnosis of PD relies on the presence of cardinal motor features and a favorable response to dopaminergic medication. However, because PD shares some major features with several other disorders, there is evidence that clinically established diagnoses may be wrong in early- and even late-stage disease. When the diagnosis is unclear, neuroimaging with PET or SPECT may help clarify it.
The biochemical hallmark of PD is the degeneration of the presynaptic nigrostriatal nerve fibers, whereas the postsynaptic side bearing the receptors remains intact, at least initially. Imaging of presynaptic terminal functions, therefore, reveals reduced radioligand uptake in the striatum of PD patients with a more pronounced decrease in the (posterior) putamen than in the caudate, and, usually, also reveals an asymmetry with more severe affection of the striatum contralateral to the limbs that are more affected, clinically. Significant correlations between disease severity and disability stages with the extent of the reduction of presynaptic terminal measures have been reported. In addition, studies in patients with early PD and those with hemi-PD (Hoehn and Yahr stage I) have concordantly shown a bilateral deficit in dopamine function. These findings were not only observed in the striatum and especially the putamen corresponding to the symptomatic limbs but also in the contralateral putamen associated with the still asymptomatic side of the body. These results strongly suggest that the aforementioned imaging techniques are capable of discriminating between subjects with early PD and healthy ones and that may also be suitable for detecting preclinical disease in sporadic and familial PD. Additionally, longitudinal imaging studies have highlighted their role in determining intraindividual disease progression. The annual rate of decline of the respective outcome measure in PD patients has been shown to range from 5% to 13% in patients with early-stage PD, whereas in those with longer disease duration, the annual decline of presynaptic outcome measures was lower (approximately 2% to 3%). In atypical PS, annual progression seems much faster; for example, in MSA patients, values of around 15% per year have been reported. Imaging of presynaptic functions has also been used in clinical trials as endpoint measures of potential disease-modifying therapies. Fluorodopa PET and 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane (ß-CIT) SPECT studies have demonstrated that the respective outcome measure showed a milder decline in patients receiving therapy with a dopamine agonist compared with levodopa. These trial results evoked an intense and somewhat controversial discussion on whether these imaging modalities may serve as useful surrogate markers for proving potential neuroprotective effects of newly developed drugs.
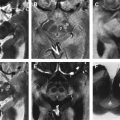
PET and SPECT with D 2 -receptor antagonists have also been extensively used to study the postsynaptic striatal D 2 -receptor availability of patients with PD. At least in earlier stages of the disease, uniformly elevated D 2 -receptor binding has been reported in the striatum contralateral to the more affected limb. Characteristically, in these cases, binding is higher in the putamen than in the caudate, and even higher in the posterior than in the anterior putamen ( Fig. 2 ). This upregulation has been interpreted as the brain’s attempt to compensate for the dopaminergic deficit related to the presynaptic nerve cell loss and may be considered characteristic of PD; whereas in aPS, the postsynaptic side is also affected by neurodegeneration and displays reduced D 2 -receptor density.
Some recent clinical trials using PET and SPECT imaging have found that a distinct subgroup of subjects with clinical criteria for (early-stage) PD presented with normal scans. These patients have been termed SWEDDs (subjects with scans without evidence of dopaminergic deficit). To date, SWEDDs have been mentioned in at least 3 large-scale clinical trials, the CALM-PD-CIT trial, the ELLDOPA study, and the REAL-PET study. Combining the data of these trials suggests that SWEDDs may be present in about 11% of included subjects (45 of 410 clinically diagnosed PD cases). This number is consistent with reported rates in the clinical literature of misdiagnosis of early-stage PD by movement disorder specialists. One possible and self-evident conclusion, therefore, would be that SWEDDs may simply reflect clinically misdiagnosed PD patients. Furthermore, follow-up data have shown that SWEDDs maintain the feature of noncompromised presynaptic functions over time and, thus, clearly differ from typical PD subjects. SWEDDs, therefore, may represent a distinct population within the mentioned clinical trials, and whether these subjects have PD or an alternative diagnosis has been questioned. Histopathologic diagnosis obtained in SWEDDs might be the clue to unravel their mystery; unfortunately it is still unavailable.
APS
MSA
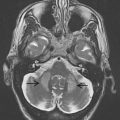
MSA is a sporadic progressive neurodegenerative disorder that may account for up to 10% of patients with ES. Clinically, MSA is characterized by varying degrees of parkinsonism, cerebellar ataxia, and autonomic dysfunction. In terms of dependency of the predominant phenotype of the motor disorder, MSA is mainly classified into a parkinsonian type (MSA-P) and a cerebellar type (MSA-C). Pathologic studies exhibit neuronal degeneration and gliosis in the basal ganglia, brainstem, cerebellum, and spinal cord. In MSA, annual progression seems much faster compared with PD; annual loss in striatal binding around 15% per year has been reported. Because MSA is characterized by a degeneration of the pre- and postsynaptic dopaminergic system, PET and SPECT investigations of both show reduced binding ( Fig. 3 ). The major difference compared to PD, therefore, is the presence of pathologic findings on the postsynaptic level, which allows one to distinguish between MSA and PD. On the presynaptic level, reliable differential diagnosis between MSA and PD is not possible.
PSP
PSP is a rapidly progressing degenerative disease belonging to the family of tauopathies. Clinically, it is characterized by parkinsonism with bradykinesia and rigidity, postural instability, and a pseudobulbar syndrome with dysarthria and dysphagia. The key feature of PSP, the supranuclear palsy of vertical gaze is rarely present at onset and usually appears later. Histopathologic findings show cell loss, gliosis, and accumulation of tau proteins in different brain regions, such as brainstem and basal ganglia, with the cortex being usually spared. Because neurodegeneration in PSP also affects the pre- and postsynaptic dopaminergic system, the PET and SPECT findings are similar to those in MSA subjects, showing a marked reduction on pre- and postsynaptic levels. Therefore, PSP patients cannot be reliably distinguished with pre- nor postsynaptic tracers from those with MSA. However, pathologic PET and SPECT findings on the postsynaptic level allow one to discriminate between PSP and PD.
CBD
CBD is an asymmetric, progressive, neurodegenerative disease characterized by cortical and subcortical involvement, with motor and cognitive dysfunction. Patients with CBD often present initially with apraxia and parkinsonian symptoms (akinetic rigid type), which usually do not respond to dopaminergic therapy. Dystonia and alien limb phenomenon are also frequently observed symptoms. Pathology reveals an asymmetric frontoparietal neuronal loss and gliosis, nigral degeneration, and variable subcortical involvement. Because corticobasal ganglionic degeneration involves the striatal presynaptic and possibly also the postsynaptic dopaminergic system, PET and SPECT studies of the latter should present with pathologic results. Concordantly, reduction in striatal fluorodopa uptake has been described and SPECT studies have also revealed a marked decrease in dopamine transporter binding. Generally, a clear asymmetry is noted with predominant affection of the striatum contralateral to clinical symptoms. Both caudate and putamen seem to be similarly affected. Reports on the postsynaptic receptor status in CBD are more rare and somewhat controversial, describing preserved and diminished striatal binding.
Other Neurodegenerative Extrapyramidal Syndromes
SCAs are a genetically heterogeneous group of autosomal dominant ataxias, which may present as pure cerebellar and various noncerebellar syndromes including parkinsonism. Imaging studies have shown decreased glucose metabolism in the cerebellum and various parts of the cerebral cortex, depending on the SCA subtypes. Decreased presynaptic dopaminergic terminal function has been reported in SCA2 and SCA3 and there are also some preliminary reports on reduced postsynaptic D 2 -receptor binding potential. Huntington disease (HD) is an autosomal dominant neurodegenerative disorder and clinically characterized by progressive cognitive impairment, neuropsychiatric symptoms, and abnormalities of movement including chorea and akinetic rigidity. Histopathologically, HD is characterized by neuronal loss and gliosis in the striatum. Accordingly PET and SPECT studies show substantial decrease in striatal perfusion, glucose metabolism, and severely compromised pre- and postsynaptic dopaminergic functions. Wilson disease (WD) is also a genetically determined disorder (autosomal recessive) that is characterized by a deficiency of biliary copper excretion resulting in pathologic copper deposition in various organs including the brain. WD goes along with extrapyramidal symptoms including parkinsonism. Similar to HD, patients with WD also present with compromised binding to pre- and postsynaptic dopaminergic targets in the striatum and show reduced striatal metabolism and perfusion, apart from the concomitant involvement of other brain areas. HD and WD are generally diagnosed clinically and genetically, with the role of functional imaging being restricted to scientific applications.
Differential Diagnosis of the Common Neurodegenerative PS
For the differential diagnosis of PD versus aPS, various nuclear medicine techniques may be applied.
As already mentioned, postsynaptic D 2 -like receptor imaging is useful in distinguishing between PD and aPS, with the former showing preserved receptors and the latter, compromised binding because of degeneration of postsynaptic fibers. A representative case example is shown in Figs. 2 and 3 . As optimized by receiver operating characteristic (ROC) analyses, reported sensitivities and specificities were 87% and 73%, respectively for SPECT and 89% and 86%, respectively for PET investigations. Combining the information of pre- and postsynaptic dopaminergic imaging marginally increased diagnostic accuracy compared with postsynaptic imaging alone. Even though reasonable results are delivered for distinguishing PD from aPS, these imaging techniques fail to further distinguish between different types of aPS reliably.
Another molecular imaging approach for distinguishing PD from aPS addresses the sympathetic denervation of the heart, rather than focusing on the brain. For this purpose, HED and MIBG may be used as PET and SPECT tracers, respectively. Autonomic abnormalities in PD have been ascribed to postganglionic sympathetic nerve dysfunction, which is depicted by the mentioned imaging techniques. In this respect, patients with PD behave differently from those with aPS, in whom normal or only mild reduction of cardiac uptake is present. Pathologically, aPS goes along with a central and preganglionic denervation that is not targeted by the mentioned tracers. Based on this principle, a reasonable number of studies have used this type of cardiac imaging for the differential diagnosis of PD and aPS. However, the diagnostic accuracy of this approach has been criticized. Overlapping values in cardiac binding of both groups raised concerns regarding the use of these techniques as the sole method for diagnostic discrimination. Limitations and confounders of the methods have to be taken into account (eg, sympathetic denervation related to other diseases, such as diabetes mellitus or several cardiac diseases).
A third option for distinguishing PD from aPS is based on the pattern analysis of brain glucose metabolism. This is the only approach that also allows one to distinguish between the various aPS. By using modern processing tools like anatomical standardization with pixelwise evaluation or discriminant and network analyses, typical metabolic patterns may be identified, which result in high diagnostic accuracies. A recent study assessing PD, MSA, PSP, and CBD on a single-case basis reported sensitivities between 85% and 96% and specificities between 60% and 92%. The study used statistical parametric mapping to create disease-related templates in which regional features were determined and used for defining or supporting specific diseases. Briefly, hypermetabolism of the dorsolateral putamen was considered indicative of PD, bilateral hypometabolism of the putamen and cerebellum, of MSA, hypometabolism of brain stem and midline frontal cortex, of PSP, and asymmetric hypometabolism of basal ganglia and some cortical areas, of CBD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree