Eye Emergencies
Matthew Flannigan
Dietrich Jehle
Juliana Wilson
INTRODUCTION
Ultrasound is a quick, noninvasive modality that improves the evaluation of emergency department (ED) patients with acute vision changes, eye trauma, and headache or altered mental status when a standard history or physical examination yields incomplete information or is impossible to perform. An adequate fundoscopic exam in the nondilated eye is challenging, almost always providing limited information due to a narrow field of view. In addition, there are a number of conditions that impair visualization of the posterior globe with direct ophthalmoscopy alone—cataracts, hyphema, hypopyon, miosis, and vitreous opacities. Facial trauma often results in substantial periorbital swelling or pain, making even a simple pupillary exam or extraocular muscle motility assessment challenging. Additionally, a thorough ocular complaint history may be compromised by altered mental status, making an ocular evaluation even more significant.
The implementation of ocular ultrasound by emergency physicians is relatively new. Since 2000 there have been numerous emergency medicine publications describing its utility in evaluating various ocular complaints (1). As a result, it has quickly risen to become one of the most important new emergency ultrasound applications.
Emergency physicians should learn a systematic approach to performing ocular ultrasound. A firm understanding of normal ocular sonoanatomy and the classic sonographic appearance of ocular pathology that requires prompt ophthalmology evaluation is necessary. While it is unrealistic to expect emergency physicians to recognize all potentially significant ocular diagnoses, ultrasound serves as an important diagnostic tool that should be correlated with the patient’s history and physical exam to yield an informed diagnosis and treatment strategy.
CLINICAL APPLICATIONS
The clinical indications for ocular ultrasound include the evaluation of acute vision changes, the evaluation of potential ocular injuries following eye or facial trauma, and the indirect assessment of increased intracranial pressure for patients with headache or altered mental status.
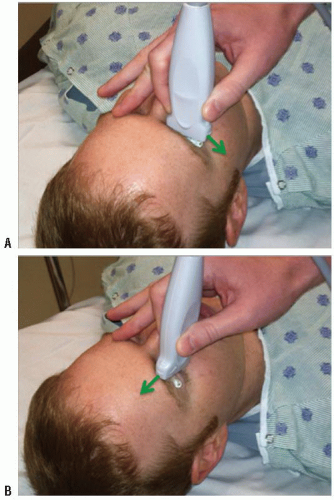
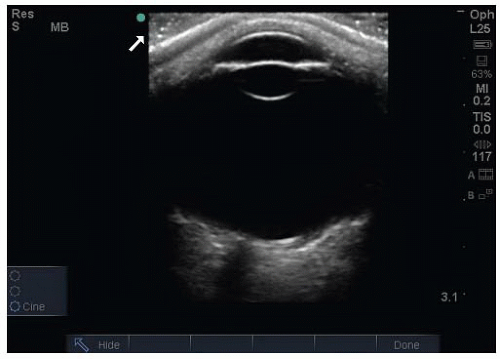
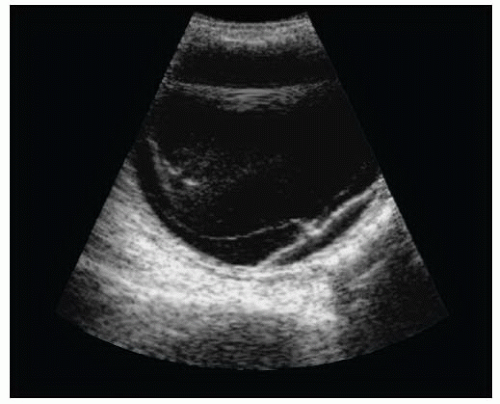
There are a number of potential safety concerns with the use of ultrasound of the orbit. Ocular ultrasound has been used safely for decades in ophthalmology (2). While there is a theoretical risk with the use of Doppler imaging modes for prolonged periods, there has been no evidence to support any significant danger to patients. Ultrasound should be performed carefully in patients with a potential occult globe rupture, as inadvertent pressure may result in the prolapse of intraocular contents. It is important for the examiner to brace their scanning hand on the patient’s nose, forehead, or cheek (Fig. 23.1) and to use a copious amount of gel to prevent pressure from being transmitted to the injured eye. This can be monitored by ensuring that an adequate gel layer is preserved at the top of the screen (Fig. 23.2).
IMAGE ACQUISITION
Equipment
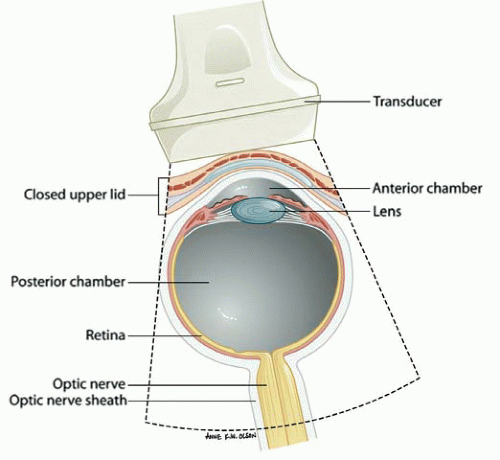
The ultrasound evaluation of the eye provides remarkable anatomic detail. It is fluid-filled, superficial, and has no inherent obstacles to impede ultrasound beam transmission. In contrast to ophthalmologists, who perform ocular sonography with a specialized transducer the size of a penlight on an anesthetized eye, emergency ocular ultrasonography is usually performed with a multi-purpose, high-frequency (7 to 15 MHz) linear array transducer on a closed eyelid (Fig. 23.3). Although unnecessary, a transparent dressing may be used as a barrier to prevent coupling gel from inducing mild eye irritation (3). Patients are typically placed in a supine to semi-upright position. The emergency ocular ultrasound assessment usually requires only standard gray scale imaging.
Imaging Technique
As with other emergency ultrasound applications, the transducer marker is directed toward the patient’s right and toward their head in the transverse and sagittal planes, respectively (Fig. 23.1). Objects closest to the transducer are represented at the top of the imaging screen (Fig. 23.2).
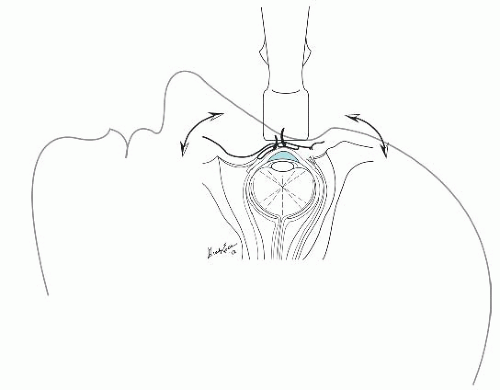
There are three basic ocular imaging planes: transverse, longitudinal, or axial. In both the transverse and the longitudinal planes, the eye is everted to scan through the sclera rather than directly through the lens (Fig. 23.4). Imaging through the lens results in increased signal attenuation, or weakening, before the ultrasound beam strikes structures in the posterior globe. In contrast, axial scanning allows for the direct visualization of the lens, macula, and optic nerve sheath.
The quality of an ultrasound image is angle dependent. Images are better defined when the incident beam is projected perpendicular to the object of interest, as a greater percentage
of sound signal is reflected back to the transducer. To facilitate complete visualization of the globe, it is necessary to fan the transducer through the entire globe. Cooperative patients can assist this evaluation by diverting their gaze into each of the four quadrants of their visual field (Fig. 23.4). To view the optic nerve sheath at a perpendicular angle, the patient should be asked to deviate their eye nasally (Fig. 23.5).
of sound signal is reflected back to the transducer. To facilitate complete visualization of the globe, it is necessary to fan the transducer through the entire globe. Cooperative patients can assist this evaluation by diverting their gaze into each of the four quadrants of their visual field (Fig. 23.4). To view the optic nerve sheath at a perpendicular angle, the patient should be asked to deviate their eye nasally (Fig. 23.5).
Dynamic Testing
Aftermovement is a description of a posterior segment lesion’s mobility following cessation of rapid eye movement. This can assist with differentiating posterior segment pathology. For example, both posterior vitreous detachments (PVDs) and retinal detachments (RDs) may appear v-shaped and may attach to the optic disc, but a detached retinal membrane will appear thicker and has less aftermovement compared to a detached posterior vitreous membrane (Fig. 23.6). In addition, blood from a vitreous hemorrhage appears to swirl while the membrane from a choroidal detachment is essentially motionless with after-movement (Table 23.1;  VIDEO 23.1).
VIDEO 23.1).
 VIDEO 23.1).
VIDEO 23.1).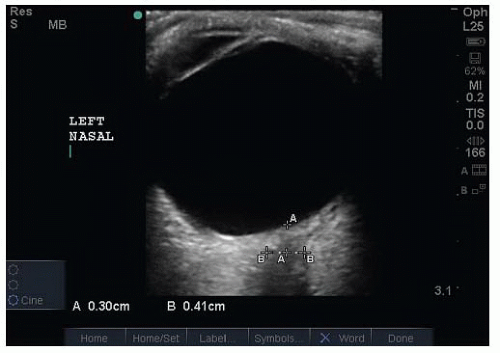 FIGURE 23.5. Measurement of a Normal Optic Nerve Sheath Diameter (ONSD). Have the patient look toward their nose to optimize the view of the optic nerve sheath. |
NORMAL ULTRASOUND ANATOMY
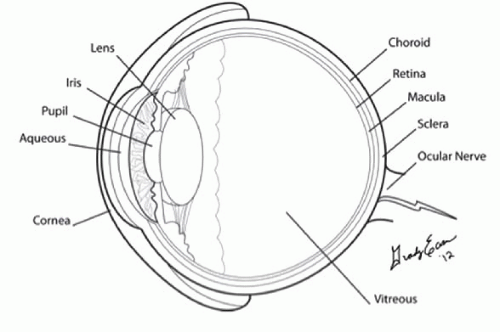
In the axial plane, the anterior chamber, iris, lens, and ciliary body can be easily identified in the near field (Figs. 23.2 and 23.3). The vitreous, in the young, healthy eye is anechoic. The retina, choroid, and sclera are indistinguishable as separate entities in the absence of pathology. The globe should have a round contour. The optic nerve sheath is identified as a hypoechoic stripe extending vertically from the posterior globe, surrounded by echogenic orbital fat (Figs. 23.5 and 23.7) (4). Extraocular muscle movement (cranial nerve) and pupillary function (Fig. 23.8) can also be assessed in real time when there is substantial periorbital soft-tissue swelling and the eyelid cannot be retracted (5).
PATHOLOGY
Lens Dislocation
Lens displacement is frequently the result of trauma, but can occur spontaneously in patients with connective tissue disorders, where it is frequently bilateral. Blunt eye trauma may stretch or rupture the zonular fibers, resulting in a lens subluxation (partial) or complete dislocation in an anterior, posterior (most common), or lateral direction (Figs. 23.9 and 23.10;  VIDEO 23.2) (6). Visual changes in an alert patient vary with the degree of lens displacement and the presence of coexistent injuries (vitreous hemorrhage, posterior segment detachments, globe rupture). Identifying a lens dislocation with ultrasound is technically uncomplicated, even for novices. Traumatic lens subluxation or dislocation requires an immediate ophthalmology consultation as coexistent ocular injuries are frequently present.
VIDEO 23.2) (6). Visual changes in an alert patient vary with the degree of lens displacement and the presence of coexistent injuries (vitreous hemorrhage, posterior segment detachments, globe rupture). Identifying a lens dislocation with ultrasound is technically uncomplicated, even for novices. Traumatic lens subluxation or dislocation requires an immediate ophthalmology consultation as coexistent ocular injuries are frequently present.
 VIDEO 23.2) (6). Visual changes in an alert patient vary with the degree of lens displacement and the presence of coexistent injuries (vitreous hemorrhage, posterior segment detachments, globe rupture). Identifying a lens dislocation with ultrasound is technically uncomplicated, even for novices. Traumatic lens subluxation or dislocation requires an immediate ophthalmology consultation as coexistent ocular injuries are frequently present.
VIDEO 23.2) (6). Visual changes in an alert patient vary with the degree of lens displacement and the presence of coexistent injuries (vitreous hemorrhage, posterior segment detachments, globe rupture). Identifying a lens dislocation with ultrasound is technically uncomplicated, even for novices. Traumatic lens subluxation or dislocation requires an immediate ophthalmology consultation as coexistent ocular injuries are frequently present.Vitreous Hemorrhage
Vitreous hemorrhage occurs when retinal blood vessels are torn or leak blood into the normally avascular vitreous space.  PEDIATRIC CONSIDERATIONS In childhood, trauma is
PEDIATRIC CONSIDERATIONS In childhood, trauma is
the most common etiology; in infants, the diagnosis of shaken baby syndrome should be explored. In adults, vitreous hemorrhage is most commonly associated with diabetic retinopathy, age-related macular degeneration, trauma, coagulopathy, retinal vein occlusion, or retinal tears (7).
In adults, vitreous hemorrhage is most commonly associated with diabetic retinopathy, age-related macular degeneration, trauma, coagulopathy, retinal vein occlusion, or retinal tears (7).
 PEDIATRIC CONSIDERATIONS In childhood, trauma is
PEDIATRIC CONSIDERATIONS In childhood, trauma is the most common etiology; in infants, the diagnosis of shaken baby syndrome should be explored.
 In adults, vitreous hemorrhage is most commonly associated with diabetic retinopathy, age-related macular degeneration, trauma, coagulopathy, retinal vein occlusion, or retinal tears (7).
In adults, vitreous hemorrhage is most commonly associated with diabetic retinopathy, age-related macular degeneration, trauma, coagulopathy, retinal vein occlusion, or retinal tears (7).TABLE 23.1 Distinguishing Characteristics of Posterior Chamber Pathology | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Vision loss is frequently described as sudden and painless. Visual field disturbances are variable depending on the degree and location of the hemorrhage: from multiple floaters or cobweb appearance (mild) to a dense smoky haze with light perception only (severe) (8). The sudden development of a vitreous hemorrhage is concerning for a retinal tear, which if left untreated, will progress to a RD.
The ultrasound appearance of a vitreous hemorrhage varies based on its age and severity. Fresh, mild hemorrhages appear as small dots or linear densities with low reflectivity; while more severe, subacute hemorrhages result in clot formation and have increased echogenicity (Fig. 23.11) (8). With aftermovement, semi-clotted blood from a vitreous hemorrhage appears to swirl ( VIDEO 23.3).
VIDEO 23.3).
 VIDEO 23.3).
VIDEO 23.3).Blood from a chronic, severe hemorrhage has a tendency to settle on the dependent portion of the globe, forming a thick, echogenic layer or pseudomembrane. This can mimic the appearance of a RD (Fig. 23.12) (8).
Posterior Segment Detachments
The retina, choroid, and sclera are tightly adherent in the normal eye and are indistinguishable as separate entities in the absence of pathology. A separation or detachment, however, between any two adjacent layers can occur for a variety of reasons (Fig. 23.7). The ability to differentiate which layer is detached requires an awareness of their classic, clinical presentation, and the ultrasonic findings associated with each detachment (8).
Posterior Vitreous Detachment
PVD is the separation of the vitreous membrane from the underlying retina. Small PVDs are uncomplicated and occur in nearly everyone, with a mean onset at age 55. Larger PVDs are often sudden in onset and are associated with vitreous hemorrhage, trauma, or inflammatory processes (8). Approximately 10% to 15% of patients with symptomatic PVDs will develop retinal tears; if left untreated, these tears result in a RD (9).
On ultrasound, PVDs appear as thin, mobile membranes (Fig. 23.13). Acutely, PVDs demonstrate a “jiggly” motion with aftermovement assessment that differs from the more rigid “shifting” mobility of a RD ( VIDEOS 23.4 and 23.5) (8,10). Chronically, PVDs become more stiff and dense, resembling the characteristics of a RD. Both PVDs and RDs can appear to have similar sonographic characteristics (Fig. 23.14); therefore, additional ophthalmologic evaluation and testing is often necessary for a definitive diagnosis (Table 23.1).
VIDEOS 23.4 and 23.5) (8,10). Chronically, PVDs become more stiff and dense, resembling the characteristics of a RD. Both PVDs and RDs can appear to have similar sonographic characteristics (Fig. 23.14); therefore, additional ophthalmologic evaluation and testing is often necessary for a definitive diagnosis (Table 23.1).
 VIDEOS 23.4 and 23.5) (8,10). Chronically, PVDs become more stiff and dense, resembling the characteristics of a RD. Both PVDs and RDs can appear to have similar sonographic characteristics (Fig. 23.14); therefore, additional ophthalmologic evaluation and testing is often necessary for a definitive diagnosis (Table 23.1).
VIDEOS 23.4 and 23.5) (8,10). Chronically, PVDs become more stiff and dense, resembling the characteristics of a RD. Both PVDs and RDs can appear to have similar sonographic characteristics (Fig. 23.14); therefore, additional ophthalmologic evaluation and testing is often necessary for a definitive diagnosis (Table 23.1).Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree