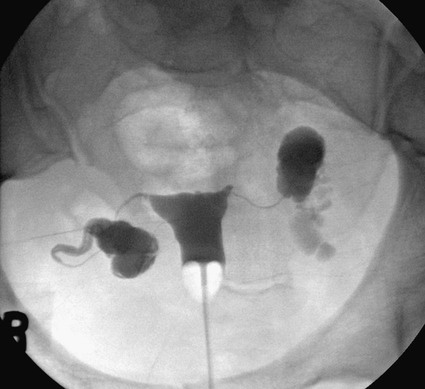
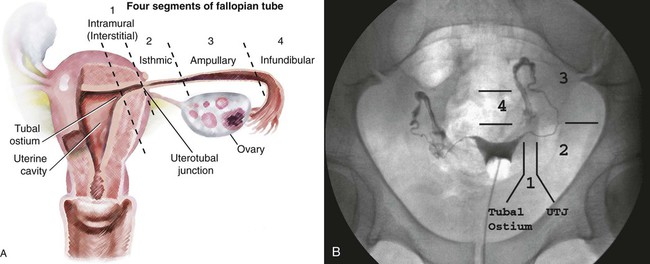
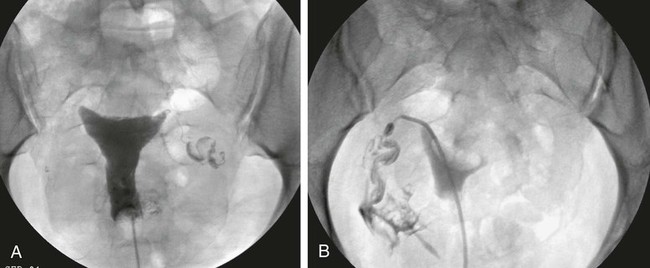
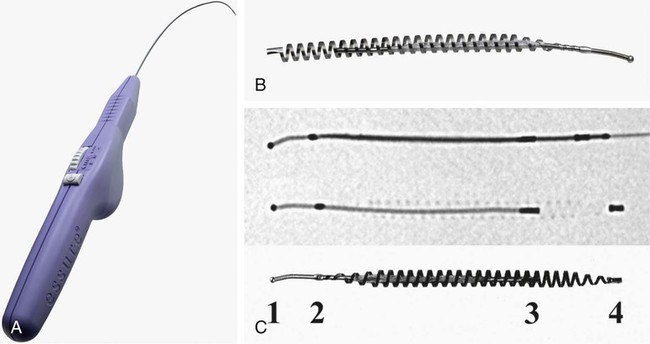
Image-guided interventions of the fallopian tubes can be divided into recanalization and embolization. Although the earliest reports of both procedures appeared in the literature in 1849,1,2 the current techniques for fallopian tube recanalization (FTR) were described in the mid-1980s3–5 and for fluoroscopic-guided fallopian tube embolization (FTE) in 2005.6 An estimated 15% of Western couples seek treatment for infertility at some point in their reproductive years.7 Selective salpingography and FTR are performed as part of the evaluation and treatment for female infertility and subfertility.4,8 Definitions of fertility are arbitrary and confusing. In reproductive medicine, subfertility refers to at least 1 year of unprotected intercourse without achieving conception, and infertility is synonymous with sterility or absolute inability to conceive.9,10 Most couples who have difficulty conceiving are not truly infertile but have decreased fertility (i.e., subfertile). Since the terms infertility and subfertility are commonly interchanged, we will consider them synonymous for the purpose of this discussion. Female subfertility can result from one or more factors and varies from one geographic and social area to another. Most developed countries have a similar distribution of causes, with tubal factors responsible for 36%, ovulatory disorders 33%, endometriosis 6%, and indeterminate causes 30%.9,10 Tubal pathology is usually secondary to pelvic inflammatory disease (PID), with Chlamydia and (less often) Neisseria gonorrhoeae the most common pathogens. The proximal (interstitial) and/or distal (fimbriated) ends of the tube are usually involved. Distal disease is best treated with laparoscopic or open microsurgery or with in vitro fertilization (IVF),10 whereas proximal tubal obstruction (PTO) is treated endoscopically or surgically. PTO can be due to a number of processes, including salpingitis isthmica nodosa, fibrosis, adhesions, amorphous debris, mucus plugs, endometriosis, polyps, and spasm.11–13 Endoscopic recanalization of PTO can be performed using a variety of methods (falloposcopy, hysteroscopy, laparoscopy, sonography, and fluoroscopy),12 but most often fluoroscopic guidance is used. The first successful transcervical fluoroscopic recanalization of a PTO was reported by Platia and Krudy in 1985.3 Thurmond and coworkers described the currently used technique in 1987 in a series of patients who underwent selective salpingography to differentiate spasm from true tubal obstruction and recanalization to treat PTO.4 When a diagnostic hysterosalpingogram (HSG) reveals obstruction of one or both fallopian tubes, the American Society for Reproductive Medicine recommends selective salpingography as the next step in evaluating infertility.14 An FTR procedure is indicated for a woman with bilateral PTO after initial HSG performed during an evaluation for infertility. At the time of the FTR procedure, repeat HSG is performed to reassess the fallopian tubes and exclude transient tubal obstruction or cornual spasm at the time of the initial HSG. Selective salpingography is performed in an attempt to clear any tubal blockage. If the PTO persists, an FTR is then attempted. If a patient has a unilateral patent tube discovered at the time of the FTR procedure, it is recommended that any PTO of the contralateral tube be treated with FTR to give the patient her best chance at conception.15,16 The two main contraindications to performing selective salpingography/FTR are pregnancy and active pelvic infection at the time of the procedure. A urine pregnancy test should be performed on the day of procedure; if this test is positive, it is then confirmed with a serum β-human chorionic gonadotropin (β-hCG) level. Before the FTR procedure, the patient should have a pelvic examination by her gynecologist, with negative gonorrhea and Chlamydia cultures documented. FTR should not be performed in cases of known distal tubal blockage (Fig. 150-1) or if both fallopian tubes are patent on HSG or selective salpingography performed immediately before FTR. If one fallopian tube demonstrates tubal patency with free intraperitoneal spillage of contrast on initial diagnostic HSG, the contralateral blocked proximal tube should be treated, especially when the patent tube has an underlying intrinsic abnormality (e.g., salpingitis isthmica nodosa, tubal polyp, or synechiae). The fallopian tube provides a conduit from the ovary to the uterus and is made up primarily of circular and longitudinal smooth muscle fibers with multiple epithelial folds. The smooth muscle layers become thinner as the tube tracks from the uterus to the ovary; the diameter of the tube also increases correspondingly. The average fallopian tube measures 11 cm (range 7-16 cm) in length and is divided into four segments (Fig. 150-2): intramural (interstitial), isthmic, ampullary, and infundibular. The intramural segment is contained in the wall of the uterus beginning at the uterotubal ostium (UTO) and ending at the uterotubal junction (UTJ). It measures 1.5 to 2.5 cm in length, with a lumen of 0.8 to 1.4 mm in diameter observed in vivo specimens.17 The narrowest point of the fallopian tube is at the UTJ. The isthmus begins at the UTJ and ends at the ampullary-isthmic junction. Its length ranges from 2 to 3 cm, and its diameter is 1 to 2 mm, making it the narrowest extrauterine segment. The ampullary segment is the most variable; it ranges from 5 to 8 cm in length, with a diameter of 1.5 mm at the ampullary-isthmic junction to 10 mm at the ampullary-infundibular junction. The infundibular segment contains the fimbria and the opening to the peritoneal cavity. HSG is first performed as a routine part of the FTR procedure to evaluate the uterus and fallopian tubes; bilateral normal patent tubes can be present in up to 20% of patients referred for an FTR. Selective salpingography through the 4F or 5F angled-tip catheter is performed for any persisting tubal blockage or suboptimal opacification of the tube (Fig. 150-3). If a PTO is present after selective salpingography, FTR can be performed with a hydrophilic 0.035-inch or 0.018-inch guidewire (with or without a microcatheter) (Fig. 150-4). A repeat selective salpingogram is performed after the FTR to document tubal patency. Endpoints for FTR are a patent tube with free intraperitoneal contrast spillage, a patent proximal tube with a distal blockage, or inability to successfully recanalize the tube. The use of water- and oil-soluble contrast agents for HSG and FTR has been extensively debated. The subsequent live birth rate after use of these agents during the procedure is the most important consideration, with the pregnancy rate second. Pinto et al. retrospectively reviewed their cases of FTR, comparing pregnancy rates for patients who underwent successful FTR.18 The two groups included use of water-soluble contrast medium only and use of water-soluble contrast medium plus addition of an oil-soluble contrast agent after confirmation of tubal patency. In 93 patients, there was no significant difference in pregnancy rates between the two groups, but the mean time to conception was 3.3 months less for the group who underwent the addition of oil-soluble contrast. However, a larger number of patients in the water-soluble-only group were lost to follow-up, with possible skewing of the data. Thus far, Pinto’s work is the only study dealing with FTR. A Cochrane review in 200519 found two randomized controlled trials for HSG using the same study groups as Pinto et al., but these demonstrated no significant benefit in pregnancy rates. Trials with other combinations of oil- versus water-soluble contrast media have shown increased pregnancy and live birth rates using oil-based contrast agents. Pinto’s work holds the most relevance because it was based on FTR using standard clinical practices. A prospective randomized trial will be useful in determining the role of oil-soluble contrast in FTR. Tubal perfusion pressure (TPP) has received new attention in the field of female infertility. TPP is measured as the resistance encountered when injecting contrast agent during a selective salpingography. Work by Papaioannou et al.20 relates poor TPP (>500 mmHg) to significantly lower pregnancy rates than a good TPP (<300 mmHg). Improved fertility was noted in women who had poor TPP at baseline and then underwent successful FTR, with TPP reduction. The concept of TPP for measuring tubal function is intriguing but has not received any attention in the radiology literature. There have been no controlled randomized trials to date to support its routine use in clinical practice. About 30% of HSGs will demonstrate bilateral PTO. Selective salpingography alone can restore tubal patency in about one third of these previously blocked tubes. For the remaining PTO after selective salpingography, FTR will be technically successful in 80% to 90% of attempts. Reported pregnancy rates after successful FTR vary between 10% and 60% depending on the individual studies’ primary patient populations.21 Overall, an average unassisted conception rate after successful FTR can be expected in the 30% to 35% range. Patients with patent normal tubes after selective salpingography/FTR have a 50% to 60% chance of tubal blockage recurring within the first year after the FTR procedure.22,23 No major complications resulting from an FTR procedure have been reported. Minor complications are uncommon, and the majority of these can be managed conservatively. Complications include tubal perforation, infection, bleeding, ectopic pregnancy, and contrast reaction. Tubal perforation occurs in 2% of cases using modern equipment and techniques.23 This is self-limited and does not require any further treatment. Infection is a rare occurrence, and the risks can be minimized with prophylactic antibiotics. The vast majority of bleeding after the procedure is mild and can last 2 to 3 days after the procedure. The risk of an ectopic pregnancy is higher for patients who have undergone FTR, but this is thought to be primarily due to underlying tubal abnormalities rather than the procedure itself. Normal monitoring for pregnancy should be performed after FTR. The possibility of a reaction to the contrast agent during the procedure exists but has not been reported. If this does occur, it can be treated with standard medications. One risk of the procedure that bears discussion is that of developing cancer or a genetic defect from the radiation exposure during the procedure. The patient should understand there is no radiation dose that is 100% safe. Papaioannou et al.24 reviewed 366 cases of selective salpingography/FTR and retrospectively calculated the median effective dose for the various combinations of procedures performed during an FTR. This dose ranged from 0.087 millisieverts (mSv) for a unilateral selective salpingogram to 0.271 mSv for bilateral salpingography and FTR. From these doses, the excess risks of cancer and genetic defects were calculated and reported as the number of cases occurring per 1 million procedures performed. These risks ranged from 4.3 to 13.5 for cancer and from 1.9 to 5.9 for genetic defects. They concluded that the overall risks associated with the radiation doses observed in selective salpingography/FTR are low. Bilateral tubal sterilization (BTS) is one of the most common methods of contraception, used by one third of women worldwide. An estimated 10.3 million women in the United States have been sterilized, and over 650,000 procedures are performed annually, making BTS the fifth most common operation.25,26 Nonsurgical alternatives to BTS using a variety of mechanical devices and/or chemical agents have been evaluated.27–35
Fallopian Tube Interventions
Fallopian Tube Recanalization
Indications
Contraindications
Technique
Anatomy and Approach
Controversies
Outcomes
Complications
Fallopian Tube Embolization
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine