Abstract
Fetal goiter is defined as an enlargement of the thyroid gland that can be secondary to thyroid dysfunction, generally hypothyroidism. It can be originated by the passage of drugs such as propylthiouracil or methimazole administered to the mother to treat hyperthyroidism. It can also be the result of the transplacental passage of stimulating immunoglobulins or antibodies against the thyroid-stimulating hormone (TSH) receptor. The enlarged thyroid gland can potentially obstruct the fetal airway and/or esophagus and so polyhydramnios can be associated. In a case where there is a suspicion of an obstruction of the fetal upper airway, an ex utero intrapartum treatment (EXIT) should be considered for the moment of delivery. Other signs associated with fetal thyroid dysfunction can be observed such as fetal tachycardia, cardiac dysfunction, or abnormalities in skeletal ossification. In cases of fetal hypothyroidism, there is a higher risk for developmental delays. Fetal growth can also be impaired when there is fetal thyroid dysfunction. Fetal ultrasound (US) is the standard for its diagnosis, but magnetic resonance imaging (MRI) could be helpful to assess the extension of the enlarged gland. Both fetal hyperthyroidism and hypothyroidism can be treated prenatally, the former by administering antihyperthyroid drugs to the mother and the latter by administering levothyroxine intraamniotically.
Keywords
hyperthyroidism, hypothyroidism, goiter, thyroid
Introduction
The prenatal diagnosis of fetal goiter was first described in 1980. Advances in prenatal imaging and fetal hormonal physiology have enabled the identification of some severe but treatable disorders in the fetus. The potential benefits to the fetus of any prenatal treatment regimen must be carefully weighed against the potential risks to the fetus and the mother.
Disease
Definition
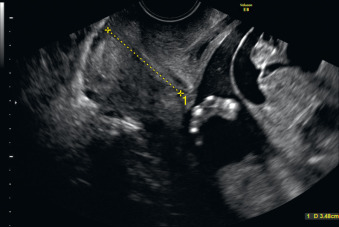
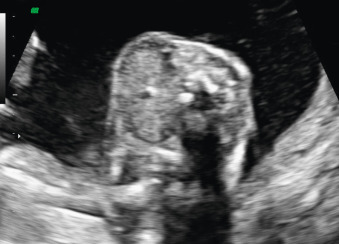
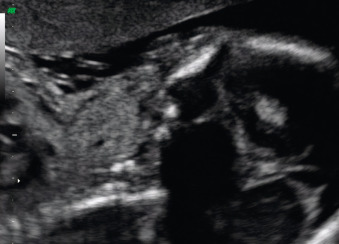
Fetal goiter is an enlargement of the thyroid gland ( Figs. 72.1 and 72.2 ). It is defined on ultrasound (US) as a thyroid circumference or diameter greater than the 95th centile for gestational age. It is frequently associated with maternal thyroid dysfunction, generally hypothyroidism.
Prevalence and Epidemiology
The prevalence of goitrous hypothyroidism is 0.2 : 10,000 to 0.3 : 10,000 live births in Europe and North America; the prevalence of the less frequent goitrous hyperthyroidism is unknown. In areas with endemic iodine deficiency, there is a higher prevalence of congenital cretinism, which may be accompanied by a fetal goiter. Iodine deficiency is still considered a major health problem worldwide.
Etiology and Pathophysiology
Congenital goiters are most commonly diagnosed in mothers with known thyroid disease, usually Graves disease. Graves disease is a common cause of hyperthyroidism that is present in 0.2% of pregnant women. This condition is treated with antithyroid drugs, including both propylthiouracil and methimazole because it passes less easily through the placenta. Reports of aplasia cutis in the fetus after first-trimester exposure to methimazole and long-term complications in the mother from propylthiouracil, particularly hepatotoxicity, have led to recommendations of first-trimester treatment with propylthiouracil then second-trimester and third-trimester management with methimazole. Fetuses of mothers with thyroid disease are especially susceptible to develop congenital hyperthyroid goiter owing to the passage of antibodies against the thyroid-stimulating hormone (TSH) receptor, also called thyroid-stimulating immunoglobulin, found in 1% of children born from mothers with Graves disease. Fetuses can also develop congenital goitrous hypothyroidism because of the transplacental passage of propylthiouracil or occasionally inhibitory immunoglobulins. Different authors have reported how maternal exposure to iodine as nutritional supplements or as a contrast used for hysterosalpingography periconceptionally could be linked to the detection of fetal goiter.
When considering all cases of congenital goitrous hypothyroidism, almost 80% are caused by thyroid dysgenesis and may be related to somatic mutations in the TSH receptor. Nearly 15% of cases are caused by dyshormonogenesis or transplacental passage of TSH receptor blocking antibodies. Dyshormonogenesis is the most frequent cause in the absence of maternal thyroid disease or iodine deficiency; it is frequently caused by recessively inherited biochemical defects in one or more steps in the pathway leading to the normal synthesis of thyroid hormones. Less than 5% of the cases are caused by hypothalamic pituitary disorders and central hypothyroidism. Endemic iodine deficiency, the use of goitrogens (expectorants with potassium iodine or povidone-iodine), and excess maternal iodine ingestion are considered less frequent causes of fetal goiters. Congenital goitrous hyperthyroidism is most frequently caused by maternal antibodies or dyshormonogenesis.
Manifestations of Disease
Clinical Presentation
A fetal goiter appears as an enlarged thyroid gland (see Fig. 72.1 ). It can be detected by a dedicated US evaluation of the fetal neck or by the complications that it may cause.
Attributable to Compression Caused by the Mass
Complications attributable to compression caused by the mass include the following:
- •
esophageal obstruction that can cause polyhydramnios and may lead to a preterm delivery;
- •
tracheal obstruction that can cause perinatal asphyxia and need for intubation; and
- •
neck hyperextension and consequent fetal dystocia.
Related to Thyroid Dysfunction
Complications related to thyroid dysfunction involve fetal hyperthyroidism and untreated congenital hypothyroidism.
- •
Fetal hyperthyroidism may cause intrauterine growth restriction (IUGR) with accelerated bone maturation, intrauterine death by cardiac failure, thyrotoxicosis, or craniosynostosis with intellectual impairment.
- •
Untreated congenital hypothyroidism is associated with impaired motor and intellectual development in the later stages of life in some affected infants. The degree of neurologic impairment has been related to the severity of fetal hypothyroidism as assessed by the age at diagnosis, the lower serum thyroxine (T 4 ) concentrations, and the delay in postnatal treatment. For this reason, an early diagnosis should be made, and subsequent treatment should be promptly started before potentially irreversible neurologic damage occurs secondary to insufficient thyroid hormone levels.
The optimal way to monitor the fetal thyroid state is debated. The proposed indirect study of the fetal thyroid status, quantifying TSH and T 4 levels in amniotic fluid for diagnosis and follow-up as a less invasive technique than cordocentesis, has limitations. First, the reference intervals for amniotic TSH and free T 4 are established only for the third trimester. Second, there is poor correlation between amniotic and fetal serum levels of both T 4 and TSH because of uncertain fetal and maternal contributions to amniotic fluid levels.
Cordocentesis is the gold standard method of diagnosis because it enables the direct quantification of fetal thyroid hormone levels. It is the most widely used method to assess fetal thyroid status. As a delicate invasive procedure, it is associated with an increased incidence of fetal bleeding, infections, fetal bradycardia, and premature rupture of membranes, and has a fetal loss rate of 0.5% to 9% ( Chapter 112 ). For this reason, some authors have relied on serial amniocentesis to determine the response to fetal treatment by amniotic fluid TSH levels and the reduction of the mass rather than with serial cordocentesis. However, amniotic fluid TSH seems less reliable as an indicator of treatment efficacy because it may remain elevated in serum while being almost undetectable in amniotic fluid. Several other reports have used serial cordocentesis as the best way to monitor the response to fetal treatment.
Imaging Technique and Findings
Ultrasound.
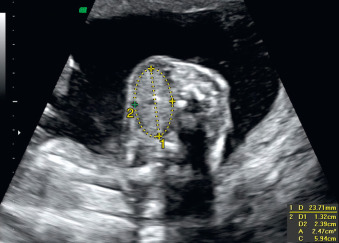
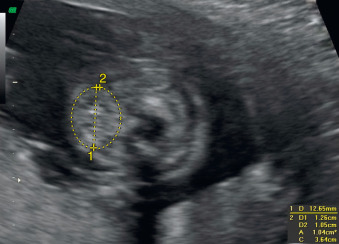
A fetal goiter appears on US as a homogeneous, echogenic, symmetric mass in the anterior portion of the fetal neck ( Fig. 72.3 ; see Figs. 72.1 and 72.2 ). When a fetal goiter is suspected, the transverse width and circumference of the thyroid gland should be measured ( Fig. 72.4 ; see Fig. 72.2 ). The diagnosis should be made when these measurements are greater than the 95th centile based on the nomograms for gestational age. Some US signs can be helpful in determining if the fetal goiter is associated with hypothyroidism or hyperthyroidism.
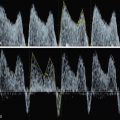
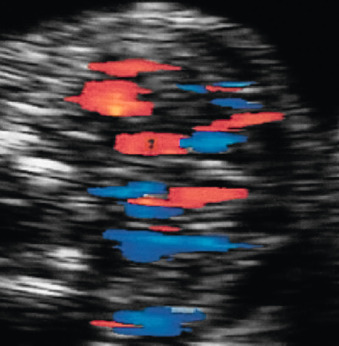
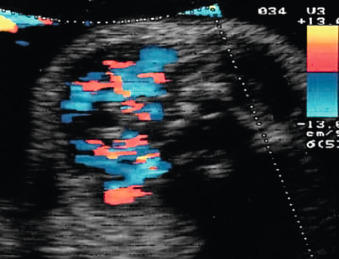
Color Doppler of the thyroid may show peripheral vascularization that reflects a hypertrophic but inactive thyroid gland, more commonly present in hypothyroid goiters ( Fig. 72.5 ). Central vascularization reveals an overactive thyroid gland, more commonly found in cases of hyperthyroid goiters ( Fig. 72.6 ). Both measurement of fetal thyroid gland and color Doppler assessment have been proposed as useful methods to monitor the response to fetal treatment. The disappearance or reduction of the mass or the Doppler signal may be associated with improvement in fetal thyroid status.
Indirect signs of fetal thyroid function include the following:
- •
Bone maturation. Accelerated bone maturation is defined as the presence of distal femoral ossification before 31 weeks’ gestation and is frequently present in hyperthyroidism. Delayed bone maturation is defined as the absence of this center after 33 weeks’ gestation and is more frequently present in hypothyroidism.
- •
Fetal tachycardia. Fetal tachycardia, defined as a continuous heart rate greater than 160 beats/min, is an indirect sign of hyperthyroidism.
- •
Fetal movements. Huel et al. described how hypothyroid fetuses in their series more frequently showed increased jerky movements, which were absent in all their cases of hyperthyroidism.
| Ultrasound Finding | Weighting |
|---|---|
| Vascularization | |
| Peripheral or absent | 0 |
| Central | 1 |
| Fetal Heart Rate | |
| Normal | 0 |
| Tachycardia | 1 |
| Bone Maturation | |
| Delayed | −1 |
| Normal | 0 |
| Accelerated | 1 |
| Fetal Movements | |
| Normal | 1 |
| Increased | 0 |
Hyperthyroidism can also be suspected when there is fetal goiter with IUGR, sometimes accompanied by oligohydramnios. In advanced stages of hyperthyroidism and hypothyroidism, heart failure with or without anasarca may be seen.
Indirect signs of thyroid goiter mass: when a congenital goiter is suspected, the amniotic fluid should always be evaluated using the amniotic fluid index because of the potential compression of the fetal esophagus. Polyhydramnios is related to goiter volume rather than etiology. When polyhydramnios is present, cervical length should be measured ( Fig. 72.7 ).