First Trimester Pregnancy
Ultrasound is the primary imaging modality used in pregnancy.1–4 In the first-trimester, pregnant patients who present with vaginal bleeding or abdominal pain, ultrasound can be used to distinguish ectopic pregnancy from threatened abortion or embryonic demise. The primary goal of emergency sonography of the pelvis in the first trimester is to identify an intrauterine pregnancy, which usually excludes the diagnosis of ectopic pregnancy.5 Secondary objectives are to detect extrauterine signs of an ectopic pregnancy, estimate the viability of an intrauterine pregnancy, clarify gestational age, and characterize other causes of pelvic pain and vaginal bleeding. In addition, sonographic detection of free fluid outside of the pelvis can help emergency physicians expedite the care of a patient with a ruptured ectopic pregnancy.6 Emergency point-of-care sonography is not intended to define the entire spectrum of pelvic pathology in early pregnancy. A follow-up comprehensive pelvic ultrasound examination may be indicated after the initial focused point-of-care examination, the timing of which is dictated by the clinical scenario.
 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS
Abdominal or pelvic pain and vaginal bleeding are common complaints during early pregnancy. Challenges to emergency or acute care physicians include making the diagnosis of pregnancy and then using available diagnostic tools to determine the etiology of the patient’s complaint.
The development of sensitive pregnancy tests has made a missed diagnosis of early pregnancy unlikely. Modern qualitative urine tests for human chorionic gonadotropin (β-hCG) have a threshold of about 20 IU/L and allow detection of pregnancy as early as 1 week postconception (3 weeks’ gestational age). False-negative urine tests may occur when the urine is highly dilute (specific gravity <1.010), and obtaining a quantitative serum β-hCG should be considered in such cases.7
Once pregnancy is recognized in a symptomatic or high-risk patient, complications of early pregnancy, particularly ectopic pregnancy, must be considered. Those patients with pelvic or abdominal pain, vaginal bleeding, dizziness, syncope, or any risk factors for ectopic pregnancy need to have the status of their pregnancy evaluated. The location, viability, and gestational age of the pregnancy are important factors in establishing a diagnosis. Other findings such as free intraperitoneal fluid in the pelvis or a pelvic mass may also impact the patient’s management.
Many diagnostic tests can be used to detect complications of early pregnancy. Serum β-hCG and progesterone levels, suction curettage, culdocentesis, and laparoscopy yield some information, but none can identify the entire spectrum of pathology like pelvic sonography. Furthermore, other imaging modalities, like CT and MRI, are not commonly used for detecting complications of early pregnancy.
The hormone β-hCG is produced by the trophoblasts during early pregnancy. Serum β-hCG levels rise exponentially in early pregnancy and can be used as a marker to date normal pregnancies. However, abnormal pregnancies have widely varying β-hCG levels, so a single level cannot differentiate a normal intrauterine pregnancy from an ectopic pregnancy or other abnormality.8
Progesterone is produced by the corpus luteum in early pregnancy and serum levels remain relatively high during a normal pregnancy. Serum levels are generally lower in abnormal pregnancies, including ectopic pregnancy, and fall with pregnancy failure. Clinicians who do not have point-of-care ultrasound immediately available have utilized progesterone levels to help differentiate between a normal pregnancy and a possible ectopic with some success.9,10 These methods, however, have not been proven to be as efficient or as accurate as protocols that incorporate initial transvaginal sonography. Reports suggest that progesterone may have a role in further categorizing patients who have an initial indeterminate transvaginal ultrasound. One study found that patients with a progesterone level ≥11 ng/mL are significantly more likely to have an early intrauterine pregnancy rather than an ectopic or an abortion (sensitivity 91%, specificity 84%).11 Another study demonstrated a progesterone level <5 ng/mL to be 88% sensitive (although only 40% specific) in detecting ectopic pregnancy in the setting of an indeterminate (nonspecific free fluid or empty uterus) ultrasound.12
Suction curettage of the uterus can provide a definitive diagnosis of an intrauterine pregnancy if chorionic villi are identified. However, this test terminates an intrauterine pregnancy, making it applicable only when termination is desired or the pregnancy has obviously failed. Because it is invasive and other tests can provide similar information, suction curettage is rarely useful in the initial emergency evaluation during early pregnancy.
Culdocentesis is needle aspiration of the pelvic cul-de-sac through the posterior fornix of the vagina. Aspiration of blood is considered indicative of an ectopic pregnancy, although blood in the cul-de-sac can be seen with an intrauterine pregnancy. However, culdocentesis is invasive and lacks sensitivity for detecting nonruptured ectopic pregnancies.5,13–15 It now has a very limited role and is recommended only when ultrasound is not available.16
Laparoscopy is an excellent test for visualizing extrauterine pelvic pathology, especially ectopic pregnancy.17 However, it does not give any information about intrauterine contents or fetal viability. Laparoscopy has been utilized less frequently because sonography is noninvasive and can provide more information.14 Laparoscopy can be used as a therapeutic tool and a diagnostic adjunct when sonography is nondiagnostic.
There are many advantages of using ultrasound in the first trimester of pregnancy. It is an ideal diagnostic tool in this setting, since it can visualize both intrauterine contents and potentially extrauterine pelvic pathology.
When used judiciously, ultrasound has no known adverse effects on the embryo and can be repeated as needed. Unlike curettage or culdocentesis, ultrasound is noninvasive and well tolerated by most patients.18 In contrast to curettage or laparoscopy, ultrasound can directly visualize the intrauterine or extrauterine location of a pregnancy.19 Unlike serum markers, ultrasound can immediately identify an abnormal pregnancy or evaluate fetal viability. Also, ultrasound can accurately measure the gestational age of a pregnancy, whereas serum markers can only give a gross estimation. Finally, patients with an ectopic pregnancy can be risk-stratified using ultrasound by estimating the size of an extrauterine mass or the amount of free intraperitoneal blood.
A disadvantage of using ultrasound in early pregnancy is that a pregnancy may not be visible between 3 and 5 weeks’ gestational age. During this time, sensitive urine pregnancy tests are positive, but the gestation is usually too small to identify, even with transvaginal ultrasound. Another disadvantage of using ultrasound is that it is both equipment and operator dependent. Clinicians who make important patient management decisions based on ultrasound results must know the limitations of each study based on who is performing the examination and what type of equipment is being used.
 CLINICAL INDICATIONS
CLINICAL INDICATIONS
Any patient who is at risk of complications of early pregnancy is a candidate for pelvic sonography. Symptoms and physical examination findings include pelvic or abdominal pain or tenderness, vaginal bleeding, dizziness, syncope, a pelvic mass, or uterine size that does not correlate with the gestational age. Risk factors for ectopic pregnancy include pelvic inflammatory disease, tubal ligation, tubal surgery, increased maternal age, intrauterine contraceptive devices, prior ectopic pregnancy, and a history of infertility.20 Classically, patients with an ectopic pregnancy present with abdominal or pelvic pain, vaginal bleeding, or dizziness, but some are relatively asymptomatic. Since no specific sign or symptom is absolute, physicians must have a high index of suspicion so that subtle presentations are not overlooked. Also, any woman of childbearing age who presents with shock of unknown etiology should have an immediate abdominal and pelvic ultrasound examination, even before a pregnancy test is completed.6
The main indication of emergency pelvic sonography in the first trimester is to differentiate an intrauterine pregnancy from an ectopic pregnancy. Point-of-care ultrasound can immediately establish one of these diagnoses in most patients with first trimester complaints.19
 Intrauterine pregnancy
Intrauterine pregnancy
 Ectopic pregnancy
Ectopic pregnancy
Emergency pelvic sonography is also useful for the diagnosis of the following conditions in the first trimester of pregnancy:
 Pregnancy loss
Pregnancy loss
 Multiple pregnancy
Multiple pregnancy
 Pelvic mass
Pelvic mass
 Ovarian torsion
Ovarian torsion
 Gestational trophoblastic disease (GTD)
Gestational trophoblastic disease (GTD)
INTRAUTERINE PREGNANCY
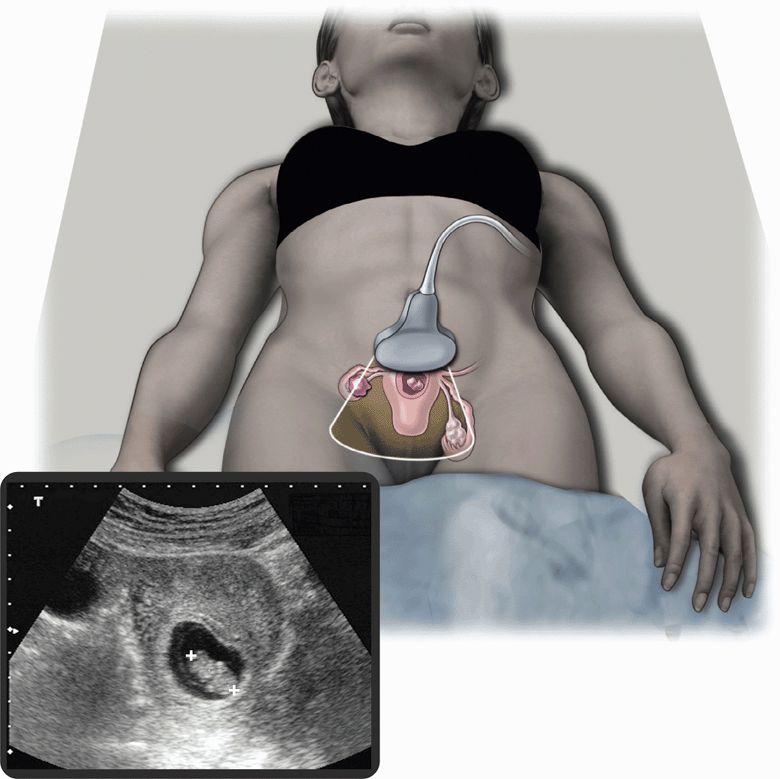
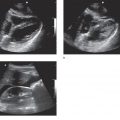
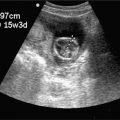
A normal intrauterine pregnancy is the most common sonographic finding during the first trimester. Pelvic ultrasound can be used effectively by clinicians with varying degrees of experience because identifying an intrauterine pregnancy is straightforward (Figure 14-1).21 In general, finding an intrauterine pregnancy virtually eliminates the possibility of an ectopic pregnancy. However, a patient undergoing fertility treatment has a significantly increased risk of heterotopic pregnancy, with both an intrauterine and ectopic pregnancy occurring simultaneously. While no further workup for ectopic is required in a low-risk patient with an obvious intrauterine pregnancy, a woman undergoing fertility treatment who has symptoms concerning for ectopic pregnancy should have more careful screening for heterotopic pregnancy.
Figure 14-1. Intrauterine pregnancy. Ultrasound techniques and findings are outlined in the corresponding sections of this chapter.
About 70% of patients who present with abdominal pain or vaginal bleeding in the first trimester will have an intrauterine pregnancy visualized with point-of-care ultrasound and will not require further testing.22 Care must be taken when using sonography between 3 and 5 weeks’ gestational age because it is easy to confuse sonographic signs of an early intrauterine pregnancy with those of an ectopic pregnancy.
Identifying an intrauterine pregnancy with cardiac activity can give patients some reassurance about the outcome of their pregnancy. Those patients with a finding of embryonic cardiac activity have a lower incidence of pregnancy loss than other patients with similar symptoms.23,24 However, clinicians should be careful not to give patients false hope about their pregnancy. Even when a normal intrauterine pregnancy is discovered, it is prudent to inform patients that point-of-care sonography is a focused examination only and will not detect fetal anomalies. Also, patients with abdominal pain or vaginal bleeding still have a significant chance of pregnancy loss.
Dating an intrauterine pregnancy is not as important as excluding an ectopic pregnancy. However, when the uterine size does not correlate with the gestational age or when the last menstrual period is unknown, sonography is indicated to date the pregnancy. This is very common because about half of all pregnant women cannot remember their last menstrual period. Pregnancy dating is simple and rapid with modern ultrasound equipment. Sonographic dating during the first trimester is more accurate than dating later in pregnancy. A few minutes spent measuring an embryo will be much appreciated by the patient’s obstetrician, especially in patients who have unclear menstrual dates or are noncompliant with prenatal care. This early measurement becomes more important when determining fetal viability after 24 weeks and also near term when the obstetrician is considering induction of labor in a patient whose uterine size does not correlate with gestational age.
ECTOPIC PREGNANCY
Ectopic pregnancy occurs in about 2% of all pregnancies in the United States.13,14,25 However, symptomatic patients who present to an emergency setting have a much higher incidence, as high as 4.5–13% in some reports.9,26–28 The incidence of ectopic pregnancy has quadrupled in the last 20 years.13 During the same period of time, the case-fatality rate for ruptured ectopic pregnancies has decreased significantly. This decrease is due to earlier diagnosis and treatment secondary to increased awareness and improved diagnostic capabilities, such as transvaginal sonography.14 Despite these improvements, a significant percentage of ectopic pregnancies are still missed.29 Also, ectopic pregnancy remains the leading cause of maternal death during the first trimester of pregnancy.30
Heterotopic pregnancy, which is a concomitant intrauterine and extrauterine pregnancy, has also become more common in the last few decades. In 1948, the incidence of heterotopic pregnancy was estimated to be 1 per 30,000 pregnancies, based on a theoretical calculation and assuming an ectopic pregnancy rate of 0.37%.31 Now that the ectopic pregnancy rate is about 2%, it is reasonable to expect that the rate of heterotopic pregnancy is higher than previously estimated. There is some suggestion that the incidence of heterotopic pregnancy may be as high as 1 in 8000 pregnancies, but this may be practice specific.31–36 The incidence is much higher in patients taking ovulation-inducing medications or undergoing in vitro fertilization (as high as 1 per 100 pregnancies).37–39
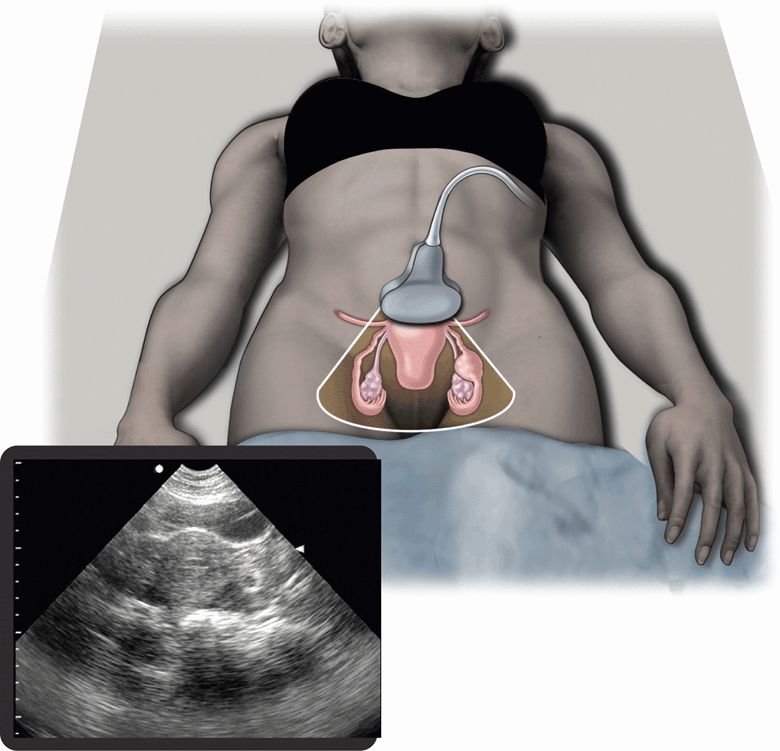
Emergency or acute care physicians have an important role in preventing morbidity and mortality from ectopic pregnancy.40 Early diagnosis of ectopic pregnancy allows conservative treatment options, like methotrexate therapy.14,41 Pelvic ultrasound is the main diagnostic modality that allows an early diagnosis to be made (Figure 14-2). Emergency physicians have been shown to be effective at performing and interpreting pelvic ultrasound examinations to rule out ectopic pregnancy. In an analysis of 10 studies with over 2000 patients, ultrasound examinations performed by emergency physicians were 99.3% sensitive for ectopic pregnancy, with a negative predictive value of 99.96%.42
Figure 14-2. Ectopic pregnancy. Ultrasound techniques and findings are outlined in the corresponding sections of this chapter.
In addition, transvaginal ultrasound is relatively straightforward to learn. A study assessed an emergency medicine residency program that conducted a single didactic lecture and 10 supervised ultrasound examinations. The residents were able to accurately identify the absence or presence of an intrauterine pregnancy in 93.3% of cases when compared to the emergency medicine ultrasound director.43
When emergency physicians perform point-of-care transvaginal sonography, pregnant patients have a shorter length of stay in the ED.44–46 Also, point-of-care pelvic ultrasound screening by clinicians is more cost-effective than ordering comprehensive pelvic sonography on every patient with a possible ectopic pregnancy.47 Most importantly, a protocol, which includes point-of-care transvaginal ultrasound by emergency physicians, has been shown to decrease the incidence of discharged patients returning with a subsequent ruptured ectopic pregnancy.22,27,48
Algorithm with Transvaginal Sonography and β-hCG Discriminatory Zone
Prior to the development of ectopic pregnancy algorithms and the widespread use of transvaginal sonography, the diagnosis of about half of all ectopic pregnancies was missed, and about half of those ruptured prior to their next presentation.27,49,50 In the 1980s, ectopic pregnancy was one of the leading causes of emergency physician malpractice suits.51,52 Algorithms that incorporate transvaginal sonography and a β-hCG discriminatory zone have improved diagnostic accuracy and reduced the incidence of patients who are discharged and subsequently present with a ruptured ectopic pregnancy.19,27
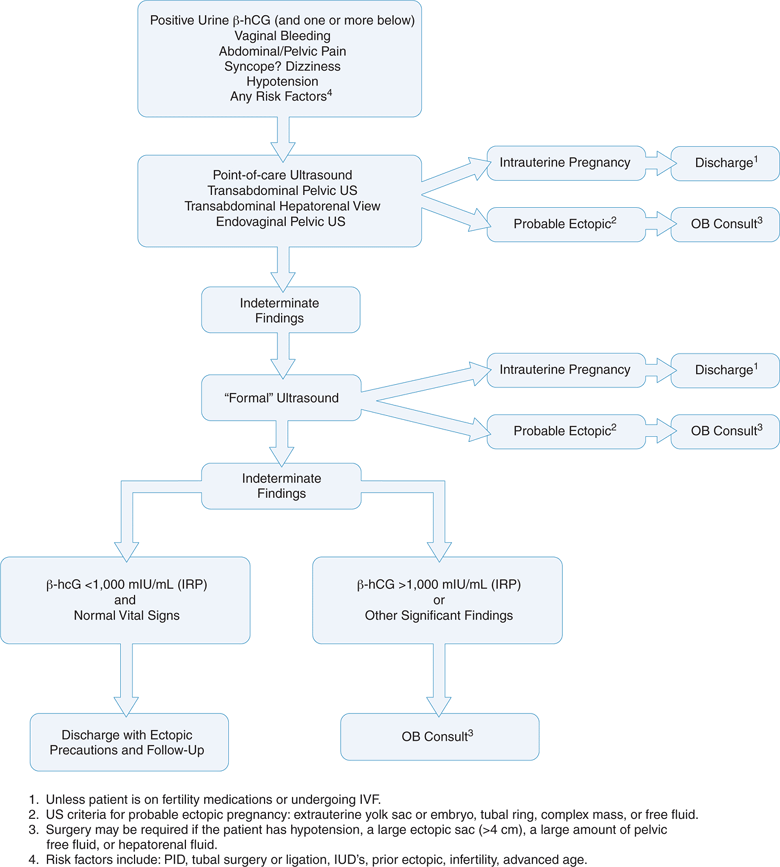
One algorithm utilizes emergency point-of-care transvaginal sonography as the initial diagnostic step for all patients at risk of ectopic pregnancy before a quantitative serum β-hCG is obtained (Figure 14-3).1,27,48,50,53–62 Transvaginal sonography can establish a diagnosis of intrauterine pregnancy or ectopic pregnancy in 75% of patients at the time of their initial presentation.19 If emergency point-of-care sonography demonstrates an intrauterine pregnancy or an ectopic pregnancy, then the workup is complete. When no intrauterine pregnancy or ectopic pregnancy is identified, then the point-of-care ultrasound examination is indeterminate, and at this point a quantitative serum β-hCG level and a comprehensive pelvic ultrasound examination should be ordered. Again, if the comprehensive study shows an intrauterine pregnancy or an ectopic pregnancy, then the workup is complete. If either the point-of-care or comprehensive ultrasound examination demonstrates nonspecific signs of an ectopic pregnancy, then the risk is very high. Therefore, this situation should be managed in the same manner as a clear ectopic pregnancy, and an obstetrics consultation should be obtained. If both sonograms show no intrauterine pregnancy and no signs of an ectopic pregnancy, then the management is more complex. Traditionally, the next step is to obtain a serum β-hCG level. Patients with an indeterminate ultrasound examination and a β-hCG level above the discriminatory zone (β-hCG >1000 mIU/mL) have a presumed ectopic pregnancy or embryonic demise and require an immediate obstetrics consultation.
Patients with an indeterminate ultrasound examination and a β-hCG level below the discriminatory zone (β-hCG <1000 mIU/mL) may have a small ectopic pregnancy, a very early intrauterine pregnancy, or embryonic demise. If hemodynamically stable and with an unremarkable physical examination, these patients can be discharged home without an obstetrics consult, but they should be given clear ectopic pregnancy discharge instructions and scheduled for close follow-up in 2–3 days for a repeat ultrasound examination and serum β-hCG level.63
Similar algorithms, incorporating point-of-care transvaginal sonography, have been shown to improve the quality of patient care and to be more cost-effective than other approaches.19,27,47,48
Quantitative Serum β-hCG and Discriminatory Zone
Serum β-hCG rises exponentially and predictably during the first 6–8 weeks of pregnancy and peaks at about 100,000 mIU/mL in a normal pregnancy. Serial β-hCG levels are useful for differentiating normal pregnancies from abnormal pregnancies. The serum level should increase by at least 66%, multiplying by 1.6, every 48 hours, and may even double between 36 and 48 hours. An abnormally slow rise in β-hCG indicates an abnormal pregnancy, either an ectopic pregnancy or embryonic demise. A normal or expected rise in β-hCG, however, does not necessarily exclude an ectopic pregnancy.
Quantitative β-hCG measurements are currently standardized in relation to the International Reference Preparation (IRP). The reference standard for all β-hCG levels discussed in this chapter is the IRP. The standard method for reporting IRP β-hCG levels is in mIU/mL. Other reference standards are referred to in the literature, and it is important to distinguish between them since they are not equivalent. The Second International Standard is roughly equal to one half of the IRP and the Third International Standard is roughly equal to the IRP. In this chapter, β-hCG concentrations are reported in relation to the IRP, in mIU/mL.
A single serum β-hCG level is not as useful as serial levels because it does not differentiate a normal early intrauterine pregnancy from an ectopic pregnancy.64 A common misconception is that a very low β-hCG level rules out ectopic pregnancy. Studies show that about 40% of ectopic pregnancies present with a β-hCG level <1000 mIU/mL and about 20% present with a β-hCG level <500 mIU/mL.19,65 In fact, patients who present with a β-hCG level <1000 mIU/mL have a higher risk of ectopic pregnancy than other patients.19,66,67 Furthermore, a low β-hCG level does not predict a benign course. Approximately 30–40% of ectopic pregnancies with a β-hCG level <1000 mIU/mL will be ruptured at the time of diagnosis.19,65,66
The “discriminatory zone” is a concept that was developed to allow the complementary use of pelvic ultrasound and a single serum β-hCG level to help determine the likelihood of ectopic pregnancy. The discriminatory zone is the β-hCG level above which an intrauterine pregnancy should be consistently visualized by pelvic sonography. Patients with a β-hCG level above the discriminatory zone who do not have an intrauterine pregnancy on ultrasound examination are presumed to have an ectopic pregnancy until proven otherwise. This is the concept that led to the use of the discriminatory zone in previous ectopic pregnancy algorithms. Older algorithms used the β-hCG discriminatory zone to limit the use of pelvic sonography since it was thought that only patients with levels >1000 mIU/mL would benefit from an ultrasound examination.50,55 When outdated algorithms are applied, however, a significant percentage of ectopic pregnancies will be missed.50
Studies clearly show the benefit of performing pelvic sonography on all patients with a possible ectopic pregnancy, regardless of their β-hCG level.19,27,61,65,66,68 Although a normal pregnancy will not be visualized when the β-hCG level is low, many ectopic pregnancies are easily identified when the β-hCG level is <1000 mIU/mL. In fact, transvaginal sonography can detect about half of ectopic pregnancies with β-hCG levels <1000 mIU/mL.19,63,65,66,69–71
Indeterminate Ultrasound Examinations
An “indeterminate” ultrasound examination in early pregnancy demonstrates no signs of intrauterine pregnancy or an ectopic pregnancy. One study attempted to subclassify patients with an indeterminate ultrasound examination based on their intrauterine findings.72 Patients with a completely empty uterus and normal thin midline stripe had a 27% chance of an ectopic pregnancy and a 10% chance of an intrauterine pregnancy. Those with a nonspecific endometrial fluid collection had a 13% chance of an ectopic pregnancy and a 25% chance of an intrauterine pregnancy. Patients with intrauterine echogenic material had a 5% chance of ectopic pregnancy and none had an intrauterine pregnancy.
Approximately 15% of patients who are evaluated for a possible ectopic pregnancy have a β-hCG level >1000 mIU/mL and an indeterminate ultrasound.19,72 Roughly 20% of these patients have an ectopic pregnancy.56,58,72,73
Management and Disposition
Patients diagnosed with an ectopic pregnancy have traditionally required surgery, which is usually a laparoscopic procedure. Although less successful compared to surgery,74,75 medical therapy has become increasingly popular, with single-dose IM methotrexate therapy being the most common regimen.76 This regimen has a success rate ranging from 64% to 94%.14,77–79 Clinical and sonographic criteria can help obstetricians decide which patients are candidates for medical therapy instead of surgery. Higher serum β-hCG levels, especially above 10,000 mIU/mL, the presence of a yolk sac, and endometrial stripe thickness >12 mm are associated with failure of methotrexate therapy.41,78,80–83 Also, an adnexal mass >4 cm in diameter, the presence of embryonic cardiac activity, a large amount of pelvic free fluid, and severe pain should be considered relative contraindications to medical management.14,84 Clinical signs of shock along with free intraperitoneal fluid outside of the pelvis, such as in the hepatorenal space, are indications of surgery and contraindications to medical therapy.6,14,30 Routinely scan Morison’s pouch for free fluid when assessing for ectopic pregnancy. Free intraperitoneal fluid identified in Morison’s pouch is predictive of the need for operative intervention. This can be rapidly performed and can significantly change management so it is recommended as a routine component of ultrasound for ectopic pregnancy.85
Patients with an unclear diagnosis of ectopic pregnancy need serial sonography and serial β-hCG levels. Obstetricians may be unwilling to initiate therapy in such patients for fear of interrupting an intrauterine pregnancy. Patients with concerning signs or symptoms and unclear or questionable sonographic findings should ideally be observed in the hospital. Repeat sonography and β-hCG level at 12–24 hours will make the diagnosis more clear. Those with minimal symptoms, no mass, free fluid, or other signs of ectopic pregnancy are safe to be discharged with early follow-up for repeat sonography and β-hCG level in 24–48 hours.13,45,48,55
The majority, up to 70% of all ectopic pregnancies, will spontaneously resolve without any treatment.86,87 Therefore, expectant management may be reasonable in selected cases. Candidates for expectant management must have minimal symptoms, a small ectopic mass, and a low β-hCG level. Excellent clinical, sonographic, and laboratory follow-up must be ensured if expectant management is attempted.
PREGNANCY LOSS
Diagnosing pregnancy loss is not as urgent as excluding an ectopic pregnancy. It is important, however, for emergency or acute care physicians to be aware of the sonographic features of pregnancy loss. It is helpful to know the risks of pregnancy loss related to specific ultrasound findings. This information will allow physicians to do a better job of counseling patients and making reasonable management plans for those with a threatened abortion.
Vaginal bleeding is a very common presentation and occurs in about 25% of all clinically apparent early pregnancies.88–90 About 40–50% of these patients will eventually be diagnosed with pregnancy loss.19,30,91–93 A threatened abortion is a significant source of anxiety for pregnant patients. Concern for the viability of the pregnancy is usually the primary reason for presentation. Pelvic sonography is very useful in patients with a threatened abortion because it provides an immediate diagnosis in about half of all patients with subsequent pregnancy loss.91 Those without a definitive diagnosis require serial pelvic sonography and β-hCG levels.
Spontaneous abortion refers to expulsion of a nonviable pregnancy from the uterus before 20 weeks’ gestational age. Microscopic identification of chorionic villi or obvious products of conception are required to make a definitive diagnosis. A completed spontaneous abortion can be diagnosed when all products of conception have been expelled. This usually occurs shortly after embryonic demise but may be delayed for days to weeks. Sonographically, an empty uterus should be seen after a completed spontaneous abortion. This finding indicates that the patient can be managed expectantly without curettage.94–96
Incomplete abortion is a nonspecific term used when a pregnancy has failed but all of the products of conception have not been expelled from the uterus. The terms embryonic demise, blighted ovum, and retained products of conception are all synonymous with incomplete abortion. Patients with an incomplete abortion may experience continued bleeding, infection, and anxiety, so it is important to make the diagnosis as soon as possible after embryonic demise has occurred. Patients with an incomplete abortion may require suction and curettage to remove retained products of conception.96,97 Sonography is the only diagnostic modality that can directly assess intrauterine contents before a curettage is performed.
The term inevitable abortion implies that expulsion of uterine contents is in progress. Patients with an inevitable abortion have an open cervical os on physical examination. Pelvic sonography may show a separated gestational sac lying low within the uterus.89 It is reasonable for physicians to use point-of-care pelvic sonography to help make initial management decisions in patients with a threatened abortion. However, it is prudent to confirm the diagnosis of embryonic demise with a comprehensive pelvic ultrasound prior to evacuation of intrauterine products. Also, it is important not to give patients false reassurance. Even when a completely normal intrauterine pregnancy is seen, they should be aware that there is still a chance of subsequent pregnancy loss.
Sonographic signs of a normal intrauterine pregnancy are reassuring and decrease the likelihood that a pregnancy will be lost.23,91,98–100 In asymptomatic patients, those without threatened abortion, the rate of first trimester pregnancy loss decreases as the gestational age increases and as more normal structures can be identified with sonography. The rate of loss after only a gestational sac is identified is 11.5%. The rate decreases to 8.5% after a yolk sac is identified and to 7.2% after an embryo (2–5 mm) is identified. When a larger embryo is seen, the loss rate is even lower: 3.3% with a 6- to 10-mm embryo and 0.5% with an embryo >10 mm. In addition, there is a 2% risk of pregnancy loss after the first trimester in pregnancies that previously appeared viable by ultrasound.99
As stated above, patients presenting with a first trimester threatened abortion have a 40–50% chance of pregnancy loss.19,30,91–93 If embryonic cardiac activity can be seen, however, the rate of subsequent pregnancy loss is lower at 15–20%.23,91 Also, as the gestational age and the size of the embryo increase, cardiac activity is more reassuring. Very early in the first trimester, when the embryo is <5 mm long, patients with a threatened abortion and cardiac activity have a loss rate of about 24%.100 Those with a threatened abortion and cardiac activity near the end of the first trimester have a very low rate of pregnancy loss.24
MULTIPLE PREGNANCY
Characterizing a multiple pregnancy (twins, triplets, etc.) is typically not in the realm of emergency medicine. However, timely pelvic sonography is indicated when menstrual dates do not correlate with the size of the patient’s uterus. In such cases, sonographic pregnancy dating and evaluation for multiple pregnancy or molar pregnancy should be performed. Also, multiple pregnancies are often an incidental finding when sonography is performed for other indications, such as ruling out an ectopic pregnancy. Regardless of the indication for the sonogram, finding a multiple pregnancy is significant since the pregnancy will then be categorized as high risk and the patient will need close follow-up with an obstetrician.
Twin pregnancies are more likely to have fetal anomalies, premature delivery, and low birth weight. Early sonographic evaluation of a multiple pregnancy is important because differentiating dichorionic from monochorionic twins is much easier during the first trimester. Fraternal (dizygotic) twins are always dichorionic and diamnionic but identical (monozygotic) twins may be dichorionic, monochorionic, diamnionic, or monoamnionic, depending on when the zygote splits. Determining chorionicity is important since monochorionic twins have a mortality rate two to three times higher than dichorionic twins. Monochorionic twins share a single placenta, so they are at risk of twin transfusion syndrome, twin embolization syndrome, and acardiac parabiotic twin syndrome. In addition, determining amnionicity is important since monoamniotic twins are at risk of cord knots, wrapping of the cord around a co-twin, or locking of twins during delivery.
When imaging a multiple pregnancy, physicians should try to record quality images that clearly show the chorionicity and amnionicity. If chorionicity and amnionicity cannot be determined, then the patient should have a comprehensive ultrasound examination within several days. Also, it is important to inform patients that about 25% of twin pregnancies diagnosed during the first trimester will become singleton pregnancies by the second trimester.101,102
PELVIC MASSES
A pelvic mass may be noted in the first trimester of pregnancy during the physical examination or routine pelvic ultrasound examination. Physicians who perform point-of-care sonography need to have some basic knowledge of pelvic masses so they can make reasonable management plans. Most pelvic masses found in the first trimester are benign and require no treatment. They all, however, require close follow-up with serial sonography because some masses are at risk of hemorrhage, torsion, rupture, dystocia, and malignancy. Surgery will be required in about 1 per 1,300 pregnancies to exclude malignancy or to deal with one of the above complications. About 3% of all masses discovered during pregnancy have malignant potential.103
In general, patients with masses <5 cm in diameter in early pregnancy are treated conservatively and followed with serial sonography. Those presenting with peritoneal signs or severe pain may need immediate surgery because of rupture or torsion of a mass. Masses that are large, cause pain, or grow rapidly may require surgery. Those containing large solid areas, solid irregular areas, papillary excrescences, and irregular septae are at higher risk of malignancy. Also, the presence of ascites, in addition to a cystic pelvic mass, increases the chance of malignancy.104 If surgery is required, then the optimal period is during the second trimester, when maternal and fetal risks are smallest.
The most common mass seen in early pregnancy is a corpus luteum cyst. The corpus luteum secretes progesterone to support the early pregnancy. A corpus luteum cyst is usually <5 cm in diameter and appears as a thin-walled unilocular structure surrounded by normal ovarian parenchyma. The appearance may vary substantially and the size may be >10 cm. Hemorrhage into a corpus luteum cyst can cause the appearance of internal echogenic debris and septae.30 Corpus luteum cysts usually regress spontaneously prior to 18 weeks of gestation.
A theca lutein cyst is an exaggerated corpus luteum and occurs in patients with very high β-hCG levels. Theca lutein cysts are commonly seen in patients with GTD and ovarian hyperstimulation from fertility medications. They appear as large multiseptated cystic masses. Theca lutein cysts usually resolve spontaneously once the abnormal stimulus is removed.
Uterine leiomyomas, or fibroids, are solid pelvic masses that are very common and may enlarge during pregnancy because of increased estrogen levels. They usually appear as relatively hypoechoic masses within the uterine wall and are sometimes confused with a simple muscular contraction of part of the uterine wall. Fibroids can have many different appearances, depending on the amount of smooth muscle and hyaline they contain and whether they have undergone hemorrhagic degeneration. They may contain calcifications or cystic areas of degeneration. Small fibroids tend to enlarge during the first and the second trimesters but larger fibroids tend to enlarge only during the first trimester.105 All fibroids tend to decrease in size during late pregnancy. Patients with multiple fibroids have a higher risk of bleeding, premature contractions, malpresentation, and retained products.104 Large fibroids located in the lower part of the uterus during late pregnancy can obstruct labor and necessitate a cesarean section.
The most common complex mass seen in early pregnancy is a teratoma, or dermoid cyst.103,104 These tumors arise from germ cells within the ovary and contain heterologous tissue like fat, skin, hair, and teeth. Sebaceous material within a dermoid can appear as a fluid-fluid level and teeth are very echogenic with distal shadowing. Dermoids are prone to torsion and rupture. Leaking of dermoid fluid can cause granulomatous peritonitis and sudden rupture can cause an acute abdomen.104
Mucinous and serous cystadenomas are ovarian epithelial neoplasms; they are the most common cystic tumors that enlarge during pregnancy.106 Both of these tumors can appear as multicystic masses. Mucinous cystadenomas usually contain multiple thick internal septations and serous cystadenomas usually appear as unilocular structures. Again, pelvic masses that have internal septations and papillary excrescences are more likely to be malignant.104
Emergency and acute care physicians typically do not attempt to characterize pelvic masses using sonography. However, these physicians will inevitably discover pelvic masses as incidental findings. When this occurs, most patients will need a comprehensive ultrasound examination and close follow-up with an obstetrician. Patients should be informed when a mass is found, and they should understand that point-of-care sonography is a screening tool and that further workup is needed.
ADNEXAL TORSION
Adnexal torsion is uncommon but about 20% of all cases occur during pregnancy.107,108 Also, most cases occur during the first trimester.105,109 Pregnant patients may be predisposed to torsion because of increased ovarian arterial flow and decreased ovarian venous flow, causing ovarian edema and enlargement. Torsion almost always occurs in the setting of an enlarged ovary or an ovarian mass; torsion rarely occurs in a normal size ovary. Ovarian hyperstimulation from fertility medications has been recognized as a risk factor for adnexal torsion because of ovarian enlargement.
Pain is the most common symptom of adnexal torsion. The diagnosis of torsion may be easily missed during pregnancy because pain may be attributed to the gravid uterus, the round ligament, or an adnexal mass. Further delay may occur because of the poor accuracy of Doppler ultrasound, which may miss up to 60% of cases of adnexal torsion.110 Also, when a cystic ovarian mass is present, blood flow to the ovary may be difficult to visualize using pulse wave Doppler, even though torsion has not occurred.
Simple gray scale pelvic sonography may be of some help in diagnosing adnexal torsion.111,112 Finding a unilaterally enlarged ovary with multifollicular enlargement or any adnexal mass makes torsion more likely. Most patients with torsion have free fluid in the pelvic cul-de-sac, probably as a result of obstruction of venous and lymphatic drainage.111,113 Finding normal-size ovaries and no pelvic free fluid makes the diagnosis of adnexal torsion highly unlikely.
Most diagnoses of adnexal torsion are delayed because of atypical clinical presentations and poor sensitivity of diagnostic modalities.114 This may be especially detrimental in pregnancy causing maternal morbidity and fetal mortality. Therefore, it is prudent to have a high index of suspicion when there is no clear etiology for abdominal, pelvic, flank, or groin pain. Also, when the diagnosis is strongly suspected, negative diagnostic studies should not deter consultation and further evaluation.115 Laparoscopy has been used during pregnancy as both a diagnostic and therapeutic modality.
GESTATIONAL TROPHOBLASTIC DISEASE
GTD is a proliferative disease of the trophoblast. It occurs in about 1 per 1,700 pregnancies in the United States but is much more common in some other parts of the world.116 GTD may occur with an intrauterine pregnancy or an ectopic pregnancy, or after a spontaneous abortion or full-term pregnancy. Most cases of GTD (80%) present as a benign hydatidiform mole. More malignant forms of GTD, invasive mole (12–15%), and choriocarcinoma (5–8%) may develop after a hydatidiform mole. Hydatidiform moles usually involve the entire placenta but a mole involving only part of the placenta can be associated with a live pregnancy.
Early in pregnancy, GTD may present with vaginal bleeding, uterine size that is too large for dates, persistent severe hyperemesis gravidarum, or early preeclampsia. Sometimes, the first clue to the diagnosis is a markedly elevated serum β-hCG level, usually >100,000 mIU/mL. It has been shown that qualitative β-hCG urine assays may be falsely negative in the setting of GTD with markedly elevated serum β-hCG.117 GTD is often discovered during routine pelvic sonography for pregnancy dating or other indications.
Ultrasound is the preferred modality for diagnosing GTD and both transabdominal and transvaginal sonography are usually diagnostic.118 The classic finding, described as having a grape-like appearance, is an intrauterine echogenic mass containing diffuse small hypoechoic vesicles. In the first trimester, GTD may not be as obvious and can be confused with an incomplete abortion. In about half of cases of GTD, a theca lutein cyst is seen in the adnexa.
Early diagnosis and prompt treatment are the key to a favorable outcome. A hydatidiform mole usually resolves completely with evacuation of the uterus. Choriocarcinoma can metastasize to the lung, liver, and brain. It is very sensitive to chemotherapy, but morbidity and mortality depend on the extent of metastases and early aggressive treatment.
 ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS
The uterus is located in the center of the true pelvis between the bladder anteriorly and the rectosigmoid colon posteriorly. The uterus is a thick-walled muscular structure that is about 6–7 cm long and about 3–4 cm in transverse and anterior–posterior diameters. It is shaped like an inverted pear and the uterine body is the widest portion. The cervix is the narrowest portion and is anchored to the posterior bladder by the parametrium. The cervix meets the vagina at the level of the bladder angle and protrudes into the anterior wall of the vagina. When the uterus is in the normal anteflexed position, the longitudinal axes of the uterus and vagina create an angle of about 90°. The fallopian tubes enter the body of the uterus laterally, in an area called the cornua. The fundus is the most superior portion of the uterine body above the cornua.
The uterine body and fundus lie inside the peritoneal cavity; intraperitoneal potential spaces exist both anterior and posterior to the uterus. The anterior cul-de-sac, between the bladder and uterus, is usually empty but can contain loops of bowel or free fluid. The posterior cul-de-sac, between the uterus and the rectosigmoid colon, is also known as the “pouch of Douglas” and it usually contains bowel loops. The posterior cul-de-sac is the most dependent intraperitoneal region when the patient is supine; therefore, it is the most common site for pooling of free pelvic fluid.
Lateral to the uterus, the peritoneal reflection forms the two layers of the broad ligament. The broad ligament extends from the uterus to the lateral pelvic sidewalls. The fallopian tubes extend laterally from the body of the uterus in the upper free margin of the broad ligament. The ovaries are attached to the posterior surface of the broad ligament. They are also attached to the body of the uterus by the ovarian ligaments and to the lateral pelvic sidewalls by the suspensory ligaments of the ovary. Normal ovaries are about 2 cm wide and 3 cm long. The ovaries are usually located in a depression on the lateral pelvic walls called the ovarian fossa. However, since the ligaments are not rigid structures, the ovaries may be seen in a number of other locations, especially in women who have previously been pregnant.
 GETTING STARTED
GETTING STARTED
While working with a stable patient in need of early first trimester pregnancy evaluation in the ED, ultrasonography of the pelvis is most efficiently accomplished immediately following the pelvic examination. This allows for uninterrupted presence of a chaperone that may have assisted with the pelvic examination. Sonographic findings of very early pregnancy and nonpregnant patients can be difficult to distinguish. Therefore, it is often advisable to wait to perform the pelvic examination and subsequent ultrasound evaluation after pregnancy status has been confirmed, usually by urine qualitative β-hCG. Occasionally patients will communicate that they are pregnant when in reality they are not. Waiting for laboratory confirmation of pregnancy status helps avoid the misuse of time and resources looking for a pregnancy that does not exist. However, if the patient’s last menstrual period suggests a sufficiently advanced gestational age, immediate transabdominal ultrasonography can confirm pregnancy and eliminate the need for urine or serum β-hCG. Immediately scan any patient who has signs and symptoms suggestive of possible ectopic pregnancy without waiting for the β-hCG level.
During the transabdominal pelvic ultrasound examination, the bladder should be full. Initially obtain a longitudinal image of the uterine midline to determine the location of the body of the uterus, the cervix, and the pouch of Douglas. Apply axial pressure to produce the best images if this can be tolerated by the patient. After imaging the long axis of the uterus, rotate the transducer transversely 90° in its long axis to find the ovaries and scan the adnexa. The ovaries are located lateral to the widest portion of the uterus in the transverse plane next to the internal iliac artery and vein. Follow the broad ligament laterally from the cornual region of the uterus to the ovary on each side.
During the transvaginal ultrasound examination, the bladder should be empty. The uterus and cervix should serve as anatomic landmarks and initially be visualized longitudinally. Because of anatomic variability, the mid-line of the uterus may not be in the same plane and consistent with the midline of the patient’s body. Ovaries are most easily visualized in the transverse plane after following the broad ligament laterally from the cornual region of the uterus. Evaluation of specific structures such as the uterus, ovaries, or adnexal masses may be enhanced by placement of the transducer tip directly over the area of interest. Just as the examiner’s hand is used to palpate structures during a routine physical examination, gentle application of axial force on the transducer may elicit tenderness and provide important diagnostic information.
 TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
Transabdominal and transvaginal sonography are complementary imaging techniques and should be used together. In general, transvaginal imaging should not be performed without also performing a transabdominal scan, but this may not be practical in a busy clinical setting. Transvaginal imaging allows the transducer tip to be placed very close to the organ of interest so that high-frequency transducers can be used to generate high-resolution images. However, transvaginal transducers have a limited field of view, and objects more than a few centimeters away from the transducer tip may not be seen. Transabdominal sonography uses lower frequency transducers so the field of view is much larger, and a better overview of pelvic structures can be obtained. The main drawback of transabdominal scanning is that the resolution is lower, so details of small pelvic structures are not as discernible, particularly ovaries and early pregnancies.
NORMAL NONPREGNANT PELVIS
Transabdominal Scanning
Transabdominal scanning is usually accomplished using a 3.5–5 MHz ultrasound transducer. The bladder is used as a window in transabdominal scanning, so it should be full to obtain optimal images. In the emergency setting, transabdominal scanning may be performed without a full bladder because it is not practical to have patients drink fluid and wait for an hour while their bladders fill. IV fluid administration will typically lead to rapid bladder filling. Quality images are usually obtained without bladder filling in thin women and those with an ante-flexed uterus. Apply gentle pressure with the transducer to produce good-quality transabdominal images without filling the bladder (See Video 16-1).
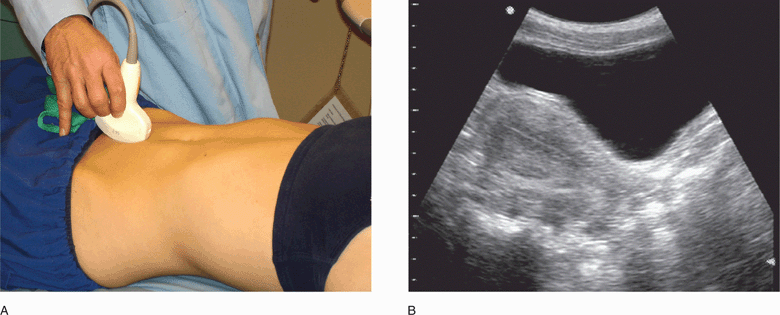
The best transabdominal view for evaluating the uterus and its contents is the standard midline sagittal view (Figure 14-4). To obtain this view, place the transducer on the abdominal wall in the midline just above the pubic bone, with the transducer indicator pointing cephalad (Figure 14-5). By convention, the indicator on the transducer should correlate with the left side of the monitor so that in sagittal images cephalad structures are on the left side. This view provides a longitudinal image of the uterus, and the entire midline stripe should be visible. The cervix is seen just posterior to the bladder angle with the body of the uterus to the left of the angle and the vaginal stripe to the right. View the ovaries by sliding the transducer laterally, with the transducer indicator still pointing cephalad, and aiming the beam toward the contralateral adnexa, using the bladder as a window. Sometimes when the bladder is very full or a large pelvic mass is present, better images can be obtained by placing the transducer directly over the adnexa.
Figure 14-4. Transabdominal midline sagittal view of the normal pelvis. Transducer position (A) and corresponding ultrasound image (B). (A, Courtesy of James Mateer, MD)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS CLINICAL INDICATIONS
CLINICAL INDICATIONS ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS GETTING STARTED
GETTING STARTED TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS COMMON AND EMERGENT ABNORMALITIES
COMMON AND EMERGENT ABNORMALITIES COMMON VARIANTS AND SELECTED ABNORMALITIES
COMMON VARIANTS AND SELECTED ABNORMALITIES PITFALLS
PITFALLS OTHER PITFALLS
OTHER PITFALLS CASE STUDIES
CASE STUDIES