Ocular
Ultrasound of the globe and orbit can be very helpful in evaluating ED and critical care patients with serious eye complaints or potentially elevated intracranial pressure. In many acute ocular conditions, the physical examination is difficult and may be unreliable. Specialized equipment and ophthalmologic expertise are frequently unavailable in the ED, especially on nights, evenings, weekends, and holidays. In these circumstances, ultrasound is more accurate than traditional examination techniques for assessing a wide variety of ocular and orbital diseases, including penetrating globe injuries, retinal detachment, and papilledema.1–4
The eye is an ideal structure for ultrasound interrogation since the anterior chamber and vitreous cavity are fluid filled. With ultrasound, the globe, orbit, and retrobulbar structures can be evaluated accurately and safely.2 While ophthalmologists typically use highly specialized ultrasound transducers, ocular ultrasound is performed using transducers readily available to emergency providers.5–8 This technology can accurately differentiate between pathology requiring immediate ophthalmologic consultation and that which can be followed up on an outpatient basis.
 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS
Physical examination incorporating ophthalmoscopic and slit lamp examination is the primary diagnostic approach to most ocular complaints. There are many situations in which the physical examination may be limited and imaging is required. Ultrasound examination of the eye is potentially useful in many situations encountered in emergency and acute care settings. Since physical examination requires a clear visual axis to examine the structures of the eye, any obstruction, such as blood in the anterior chamber or vitreous, obscures visualization and limits physical examination. Ultrasound allows imaging beyond the obstruction. There is little attenuation of the ultrasound signal. Detailed, high-resolution images of posterior structures can be obtained even when direct visualization is difficult or impossible.
Situations in which direct visualization of intraocular structures may be difficult or impossible include lid abnormalities due to facial trauma, severe edema, subcutaneous air, or previous surgeries. In cases of facial trauma and swelling, it may be difficult to assess the eye without significant manipulation, which may be painful and even harmful if there is globe perforation. Visual axis obstruction can also occur in the presence of corneal scars, cataracts, hyphema, or hypopyon, or with vitreous hemorrhage. Furthermore, normal conditions such as miosis make visualization of the retina difficult without pharmacologic agents.
Ultrasound may also be helpful in situations where physical examination alone is inadequate. An example is peripheral retinal detachment. Patients presenting with a history consistent with retinal detachment may have an unremarkable ophthalmologic examination, and performing an examination with a dilated pupil is not always feasible. Ultrasound allows for visualization of the entire retina without dilation of the pupil.
CT is frequently employed to evaluate the globe after trauma. CT is highly sensitive for orbital fractures, foreign bodies, and retrobulbar hematomas. Fine-cut CT scans with 2-mm sections are able to localize foreign bodies as small as 0.7 mm.9 In contrast, ultrasound has been demonstrated to have a slightly lower sensitivity but a comparable positive predictive value for detecting similar size metal foreign bodies in a porcine model.10 Patients with a potential foreign body and a negative ultrasound examination will require an orbital CT scan.
 CLINICAL INDICATIONS
CLINICAL INDICATIONS
The clinical indications for ocular ultrasound are as follows:
 Eye trauma
Eye trauma
 Acute change in vision
Acute change in vision
 Headache, head trauma, or altered mental status (potentially elevated intracranial pressure)
Headache, head trauma, or altered mental status (potentially elevated intracranial pressure)
EYE TRAUMA
Trauma is one of the leading causes of unilateral loss of vision in the United States and accounts for an estimated cost of $200 million per year.11 Vision-threatening injuries include retrobulbar hematoma, retinal detachment, lens dislocation, traumatic optic neuropathy, and globe injuries.12 The most common presentation for a vision-threatening injury is blindness after the injury. However, vision loss may occur gradually because of unrecognized trauma or the patient may not be able to report vision loss due to lid swelling or alteration in mental status. As a consequence, ocular injuries are often missed in these patients. A retrospective review of trauma patients with potential ocular injury demonstrated that nonophthalmologists frequently missed or underestimated potential eye trauma, diagnosing only 72% of eye injuries and referring only 27% for ophthalmologic evaluation.13
Significant swelling of the periorbital tissues can be encountered with midface or craniofacial fractures, injuries that increase the risk of concomitant ocular injury. One study found that of 283 patients presenting with facial fractures, 71 had an ocular injury, with 32 (12%) suffering a serious ocular injury.14 While it is ideal for all facial trauma patients with suspected eye injury to have an examination performed immediately by an ophthalmologist, this is commonly not feasible. There are often more serious and life-threatening injuries that require emergent evaluation and treatment.
Ocular ultrasound can be performed at the bedside. It is noninvasive, so there is little risk for exacerbating an injury when it is performed correctly. Ocular ultrasound is useful even when medication, drugs, or hypoxia alter pupillary function. This is important in the initial clinical assessment of the eye, where pupil size, reaction to light, and the presence or absence of a relative afferent papillary defect are assessed.12
Retrobulbar hemorrhage can be diagnosed clinically when a significant hematoma is present.15 However, this vision-threatening emergency often goes unrecognized, especially in trauma victims with a decreased level of consciousness, and delayed diagnosis may result in irreversible damage to the optic nerve. CT typically reveals stretching of the optic nerve and a tented posterior sclera. Retrobulbar hematoma may cause damage to the optic nerve either through direct compression leading to ischemia or through traction by propelling the globe forward and stretching the nerve. The hematoma occurs as a result of bleeding within or around the cone formed by the extraocular muscles. This cone of muscles combined with the bony orbit forms a compartment in which ongoing hemorrhage leads to elevated intraorbital pressure. As the pressure rises, compression of the ophthalmic and retinal vessels can occur, resulting in ischemia and ultimately blindness.12 Any potential injury must be treated rapidly because irreversible damage may occur after only 60 minutes of ischemia.16
An open globe injury is defined as a full thickness wound involving the corneoscleral wall of the eye. This type of injury is typically caused either by blunt force, particularly to the anterolateral part of the orbit, or due to laceration by a foreign body.12,17 While some open globe injuries are obvious with vitreous extrusion, a significant proportion are not readily apparent. Clues to an open globe injury include bloodstained tears, lid lacerations, presence of a significant subconjunctival hemorrhage, or hyphema. However, none of these are pathognomonic for globe rupture. Furthermore, in cases involving small high-velocity projectiles, there may be no external signs of perforation.12 Standard CT evaluation for open globe injury has been shown to have a sensitivity and specificity of 75% and 93%, respectively.18 Ultrasound sensitivity is similar to a standard CT scan. The best test for identifying globe injury is thin slice CT (2-mm) with reconstructions, which can identify subtle findings such as scleral discontinuity.
Thin slice CT is also the test of choice for intraocular foreign body localization.9 Ultrasound has limitations when used for the detection of intraocular foreign bodies. In animal models, the sensitivity and specificity were 87.5% and 95.8%, respectively.10 One study compared CT with ultrasound in a prospective study of patients with opaque intraocular foreign bodies. Ultrasound had complete concurrence with surgical or clinical follow-up in 90% of 61 cases. The study concluded that ultrasound was useful, even though CT was more accurate in detecting the intraocular foreign bodies, because ultrasound was superior to CT in demonstrating intraocular damage associated with the foreign bodies.
ACUTE CHANGE IN VISION
Acute change in vision is a fairly common complaint in emergency and acute care settings. Symptoms may include floaters, flashing lights, double vision, and even complete blindness. Although these symptoms may be indicative of nonocular problems, they require rapid attention to exclude such processes as lens dislocation, vitreous hemorrhage, retinal detachment, and vitreous detachment.
Lens dislocation is a condition frequently caused by blunt trauma. In a series of 71 consecutive patients presenting with ocular trauma, 12 were noted to have a lens dislocation.19 Lens dislocation can also occur without trauma and be idiopathic or hereditary (Marfan’s syndrome). Ultrasound easily identifies the lens due to its anterior location. Using ultrasound, the clinician is able to evaluate the lens-supporting structures to determine whether the lens is subluxed or completely dislocated. Subtle lens subluxation can be a difficult diagnosis, even for experienced clinicians.
The incidence of spontaneous vitreous hemorrhage is about 7 cases per 100,000 people.20 Proliferative diabetic retinopathy, posterior vitreous detachment with or without a retinal tear, and retinal detachment are the most common causes. Symptoms of a spontaneous vitreous hemorrhage typically include floaters or clouded vision. Other symptoms such as flashes of light may be present, but these symptoms are usually due to the underlying cause of the hemorrhage, such as retinal detachment. As a vitreous hemorrhage ages, a membrane may form with attachments to the retina. When this membrane contracts, a retinal detachment may occur, usually weeks after the initial hemorrhage. Ultrasound allows for visualization of the hemorrhage as well as the potential cause. In fact, there is no other imaging modality that can reliably ascertain the anatomic position of the retina.20
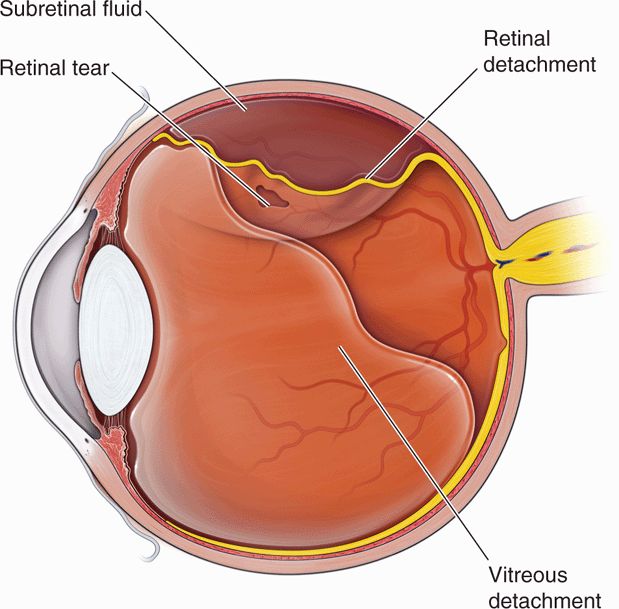
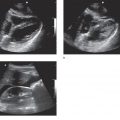
Retinal detachments and retinal tears (Figure 19-1), which may be a precursor to detachment, are common causes of vitreous hemorrhage. Both represent a separation between the retinal sensory and the pigment layers. There are three types of retinal detachment: rhegmatogenous, tractional, and exudative. Most cases of rhegmatogenous retinal detachment are associated with a posterior vitreous separation; the detachment is caused by fluid seeping into a break in the sensory layer of the retina. Tractional retinal detachments occur when fibrous membranes in the vitreous pull the retina from the underlying retinal pigment epithelium, and they are typically seen with proliferative diabetic retinopathy or as a common sequela of aging. However, this condition is also associated with retinopathy of prematurity, sickle cell retinopathy, and prior vitreous hemorrhage. Exudative retinal detachment occurs with inflammatory, infectious, or neoplastic conditions that disturb the blood–retina barrier. This allows fluid to collect underneath the layers of the retina causing a separation. In most cases of retinal detachment, patients will complain of flashing lights, floaters, or a curtain-like vision loss.21 As the retinal tear progresses, retinal vessels may tear, producing a vitreous hemorrhage. This is a common association and is why patients with retinal detachments typically complain of floaters prior to the onset of peripheral or total vision loss.
Posterior vitreous detachment is a separation of the vitreous humor from the retina (Figure 19-1). The separation is painless and usually abrupt; patients complain of new floaters in conjunction with the onset of flashing lights. The clinical history can be similar to retinal detachment as patients with both problems complain of flashing lights at onset. This entity is a common presentation for acute visual change in the urgent care and emergency settings as it is a normal consequence of aging. The condition occurs often in older adults and over 75% of those greater than age 65 develop it.22 Vitreous detachment often has a benign clinical course that does not significantly threaten visual acuity; however, in approximately 15–30% of cases of vitreous detachment, a retinal hole is created that may lead to retinal detachment. Posterior vitreous detachment can also be associated with vitreous hemorrhage.23 Ultrasound is the modality of choice for evaluating the retina and vitreous. Ultrasound may be the only method for detecting posterior vitreous detachment and is also more accurate for identifying potential associated complications (retinal detachment or hemorrhage).
HEADACHE, HEAD TRAUMA, OR ALTERED MENTAL STATUS
Headache, head injury, and altered mental status are common presentations in the ED. These complaints may be associated with elevated intracranial pressure. While many modalities are available to evaluate potential elevated intracranial pressure, each has significant limitations. In the acute trauma patient, evaluating the presence of papilledema with an ophthalmoscope is difficult. Furthermore, papilledema can take hours to develop. CT is the most common initial diagnostic modality, but it may be unavailable or obtained early in the patients course before elevated intracranial pressure develops. When unstable patients are taken directly to the operating room for abdominal injuries, there may not be time for a head CT. In these patients, ocular ultrasound can provide a method for grossly assessing the intracranial pressure. In addition, multiple examinations can be readily performed on the same patient with an evolving clinical picture.
A direct communication between the subarachnoid space of the ventricles and the optic nerve sheath has been described in cadavers and an animal model. In an experimental model using rhesus monkeys, change in the optic nerve sheath diameter in response to changing intracranial pressure was demonstrated by varying the pressure in balloons placed in the subarachnoid space.24 Multiple clinical studies have demonstrated this effect in actual patients. One study comparing CT to ultrasound measurement of the optic nerve sheath diameter in patients with suspected intracranial hemorrhage showed a sensitivity and specificity of 100% and 95%, respectively.25 This procedure has also been described in pediatric patients.26
 ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS
The globe is an oblong structure with a mean vertical diameter of 23.5 mm and mean anteroposterior diameter of 24 mm. The eye is embedded in the orbit and covered by the eyelids. On ultrasound evaluation, the surrounding facial bones appear as bright reflectors with deep posterior shadowing. The lid is echogenic and divided by the hypoechoic tarsal plate.
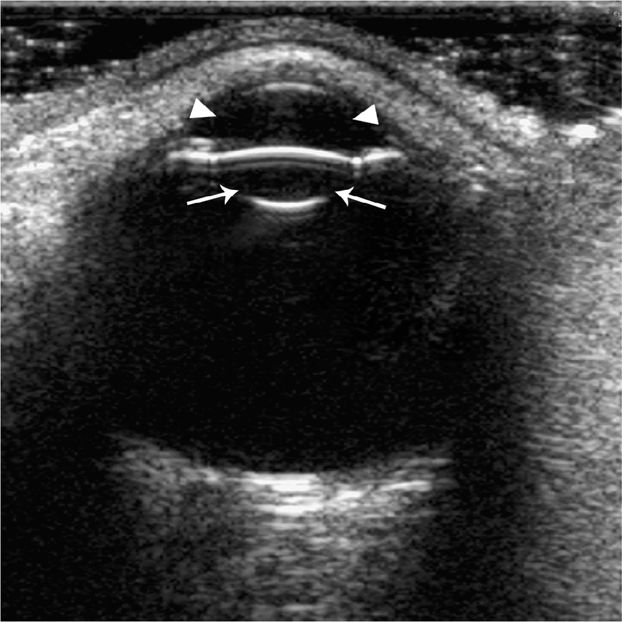
The eye is divided into anterior and posterior segments. The anterior segment consists of the cornea, anterior chamber, iris, posterior chamber and lens. The posterior segment consists of the structures posterior to the lens; the vitreous chamber, retina, choroid, and optic nerve. The cornea appears as a thin, hyperechoic structure attached to the sclera at the periphery. The sclera is a dense membrane to which the extraocular muscles attach, but is indistinguishable from the lateral structures of the eye by ultrasound. Anechoic aqueous humor fills the anterior chamber, and echoes in the anterior chamber are seen only in pathologic states. The iris and pupil can often be visualized between the anterior chamber and the lens. The lens appears as a hyperechoic reflector, which is concave, and may show reverberation artifact at the anterior surface (Figure 19-2).
Figure 19-2. Normal ultrasound of the eye. The anterior chamber (arrow heads) and the lens (arrows) are clearly seen. The vitreous appears black.
The posterior segment comprises the majority of the globe, about 80%. It contains the vitreous body, which consists of a colorless, structureless transparent gel, approximately 99% of which is water. The normal vitreous body is echolucent (dark on ultrasound); however, ultrasound artifacts may occasionally be seen.
The posterior layers of the eye consist of the retina and choroid, which are bounded by the sclera. The retina is the neural, sensory stratum of the globe. It is very thin, varying from 0.56 mm near the optic disk to 0.1 mm anteriorly. Its anterior surface is in contact with the vitreous body and the posterior surface is strongly adherent to the choroid. Near the center of the posterior aspect of the retina is the oval macula, where visual resolution is the greatest. At the macula, the retina is only a few cell layers thick. The choroid is a thin, highly vascular membrane lining the posterior globe. It is firmly adherent to the sclera and is thicker posteriorly where it is penetrated by the optic nerve. Its internal surface is firmly attached to the pigmented layer of the retina. On ultrasound interrogation of the normal eye, the three posterior layers (retina, choroid, and sclera) blend into one homogeneous structure. If separation occurs as with retinal detachment, the layers may be distinguished from one another.
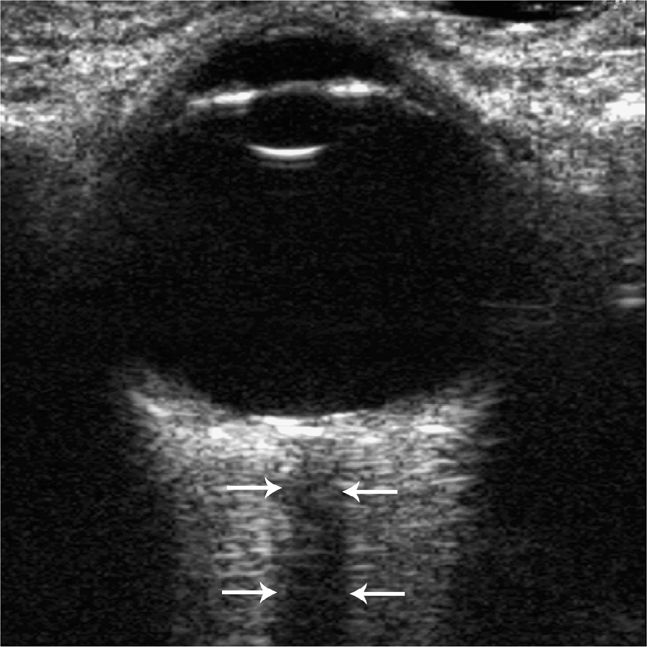
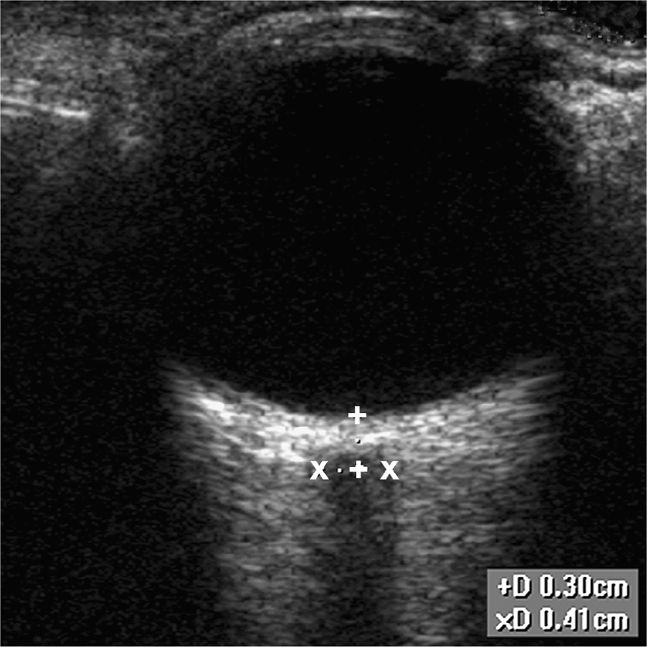
The optic nerve and sheath may be seen posterior to the globe, traveling toward the optic chiasm. The nerve appears homogeneous on ultrasound, with low internal reflectivity (Figure 19-3). This is in contrast to the more reflective sheath.27 Measurement of the sheath is made 3 mm posterior to the globe (Figure 19-4). Normal measurements vary by age from 5 mm and less in adults, 4.5 mm in children (1–15 years), and 4 mm or less in children less than 1 year of age.25–27 Since the optic nerve sheath is connected to the subarachnoid space and is easily distensible, conditions that elevate the intracranial pressure lead to dilation of the optic nerve sheath.
Figure 19-3. The optic nerve is seen posterior to the globe and is hypoechoic (arrows). Minimizing the gain is advisable to decrease echoes that can result in difficulty defining borders of the optic nerve sheath.
Figure 19-4. Normal measurement of the optic nerve sheath is shown. The measurement should take place 3 mm behind the globe.
Arterial and venous structures of the eye can be identified using color Doppler. The largest artery in the orbit is the ophthalmic artery, which runs parallel to the optic nerve.28 The central retinal artery is located in the anterior part of the optic nerve shadow. The central retinal vein is found near the central retinal artery and is distinguishable from the central retinal artery with pulsed wave Doppler (PWD) examination.29
 GETTING STARTED
GETTING STARTED
Patient positioning will vary, with trauma patients typically supine while other patients may be partially reclined or upright. Patients with a potential penetrating injury may be reclined at 45°. Make the patient comfortable with oral or parenteral medications. When a ruptured globe is suspected, consider administering antiemetics to prevent retching and a resultant rise in intraocular pressure. Topical anesthetic and cycloplegic medications are not necessary for sonographic examination of the globe.
Set the ultrasound machine with the ocular presets, which will help limit the amount of energy exposure to the ocular tissue. Always practice the ALARA (as low as reasonably achievable) principle to ensure that the total ultrasound energy is maintained below a level at which bioeffects are generated while diagnostic information is preserved. Use a linear array transducer in the 7.5–15 MHz frequency range (the same transducer utilized for line placement, soft tissue examinations, and musculoskeletal studies). Higher transducer frequencies may produce better images, but decreased penetration may limit visualization of retro-orbital structures. Use an endocavity transducer if a linear transducer is not available1; however, manipulating an endocavity transducer over the eye can be awkward and imaging is somewhat limited. Color and spectral Doppler capabilities allow for the evaluation of orbital vasculature and may be helpful in specific cases. An ocular or small parts setting is available on most machines. Other presets such as thyroid, musculoskeletal, and superficial settings may also be used. Sterile gel is typically not required but can be utilized.
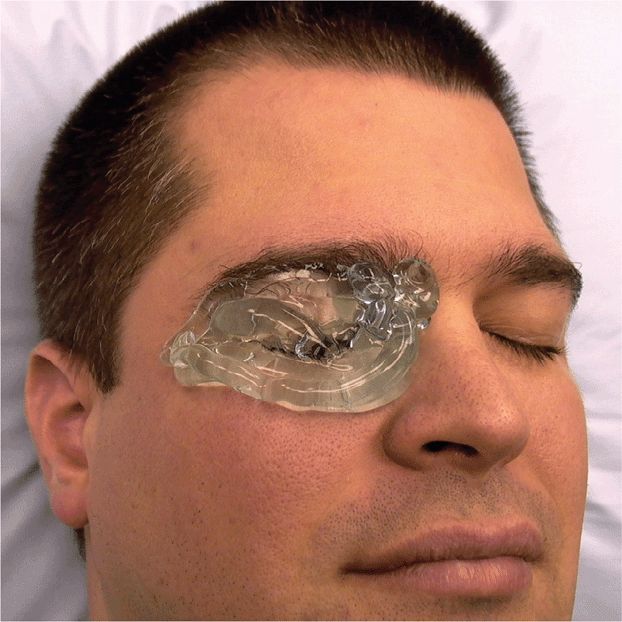
The amount of ultrasound gel needed for ocular ultrasound depends on the possible pathology. If globe rupture or pathology in the very near field is suspected, then use a larger amount of gel to fill the preorbital space. With the eyelid closed during the examination (Figure 19-5), allow the transducer to “float” on the ultrasound gel while not touching the eyelid. If the lid is not touched and no pressure is transmitted to the globe, the possibility of vitreous extrusion from a perforated globe is minimal. The space between the transducer and the eyelid can be clearly visualized during the ultrasound examination as an echo-free space between the top of the screen and the lid, and confirms that no pressure was applied to the globe during the examination (Figure 19-6A). If globe rupture is suspected, it is advisable to have the patient keep both eyes closed so they are less likely to inadvertently open the eye being examined. If a ruptured globe is not suspected, then very light contact with the eyelid is permitted, but will still require a moderate amount of gel. The gel is nontoxic to ocular structures; however, removal of gel from the preorbital space may be difficult in a patient with a painful eye condition. Further, some patients complain of a burning sensation if the gel comes in contact with the cornea. When the transducer touches the eyelid, near-field structures are too close to the transducer and are not well visualized, which is in contrast to when the transducer is “floated” within the ultrasound gel (Figure 19-6B).
Figure 19-5. Place a generous amount of gel on top of the closed lid in the patient with suspected globe rupture. This much gel allows the clinician to make no direct contact between the transducer and the eyelid itself.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS CLINICAL INDICATIONS
CLINICAL INDICATIONS ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS GETTING STARTED
GETTING STARTED TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS COMMON AND EMERGENT ABNORMALITIES
COMMON AND EMERGENT ABNORMALITIES COMMON VARIANTS AND SELECTED ABNORMALITIES
COMMON VARIANTS AND SELECTED ABNORMALITIES PITFALLS
PITFALLS CASE STUDIES
CASE STUDIES