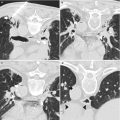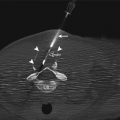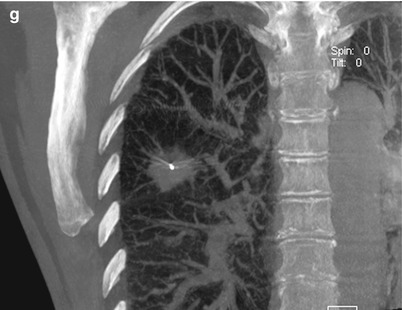
Fig. 6.1
Fluoroscopic-guided lung biopsy. A 63-year-old woman with newly diagnosed right upper lobe lung lesion was referred for biopsy. Axial CT image of the chest (a) shows a 2.3 cm mass in the right upper lobe. Biopsy was performed under real-time fluoroscopic guidance (b). The C-arm was angled caudal approximately 10° to place the lesion (arrowheads in b) in between the anterior ribs. The needle was then advanced parallel to the x-ray beam into the lesion under real-time fluoroscopy. Two orthogonal images (right anterior oblique [c] and left anterior oblique [d]) were acquired to confirm the depth of the biopsy needle and appropriate position of the biopsy needle within the mass (arrowheads). C-arm cone-beam CT images were acquired and reconstructed in axial (e), sagittal (f), and coronal (g) planes to confirm the position of the biopsy needle within the tumor. Pathology showed non-small cell lung cancer
Unlike fluoroscopy, which provides real-time guidance, CBCT images require up to 8 s for acquisition and up to 1 min for reconstruction and thus, CBCT alone cannot be used for real-time needle guidance. Dedicated needle path-planning software has been developed to complement CBCT for needle guidance. The needle trajectory is planned in the 3D dataset, which is then coregistered with real-time fluoroscopy. The desired needle insertion site and the calculated trajectory are displayed over real-time fluoroscopic images, guiding accurate needle placement [11, 12].
Advantages
The main advantage of fluoroscopy over other imaging modalities for guiding percutaneous biopsies is the real-time nature of the technology. In addition, fluoroscopy is readily available, is relatively inexpensive compared with CT and MRI, and provides easy access to the patient during the procedure. If available, CBCT can be used to confirm the exact position of the biopsy needle that is inserted under real-time fluoroscopic guidance. When used with image-overlay software, CBCT allows accurate localization and targeting of certain structures and lesions.
Disadvantages
Fluoroscopy is limited to imaging in a single plane. Fluoroscopy flattens a volume of tissue to a single two-dimensional image and is not capable of characterizing tissue traversed by the needle as it travels to the target. Fluoroscopy also has limited soft-tissue contrast resolution. Therefore, only tumors or structures that have density that is markedly different from that of their surroundings can be targeted for biopsy under real-time fluoroscopy. For example, a relatively small (about 2 cm) solid tumor within an aerated lung has a density that is adequately different from its surrounding tissue to be visualized under fluoroscopy, whereas a large soft-tissue mass in the retroperitoneum may not be adequately detected or targeted with fluoroscopy.
CBCT and image overlay may help alleviate some of fluoroscopy’s shortcomings in certain cases; however, at the time of this writing, the resolution of CBCT images was suboptimal compared with other cross-sectional imaging modalities used to guide complex biopsies of deep-seated structures within the abdomen or retroperitoneum [13].
Applications
Fluoroscopy is a viable imaging modality for guiding biopsies of certain lesions of the lung or bone. Fluoroscopy is also the preferred imaging modality for guiding endoluminal biopsies of bile ducts and ureters and for guiding transjugular liver and renal biopsies.
Although CT is the modality of choice for guiding percutaneous lung biopsies [14], lesions in the lower lobes are affected by respiratory motion and can be difficult to target with CT. CT fluoroscopy, with or without respiratory control, can help target some of these lesions [15, 16]. However, some radiologists prefer fluoroscopy-guided lung biopsies for lesions that can be easily identified on chest radiography [4]. A review of diagnostic CT images can help select patients appropriately and can help determine the optimal patient position and biopsy approach [17].
Sclerotic (Fig. 6.2) and lytic (Fig. 6.3) bone tumors often create adequate changes in density under fluoroscopy to allow visualization and targeting for biopsy [3, 18]. Transpedicular vertebral body biopsy is a simple and effective sampling technique when the vertebra is diffusely involved by a pathological process [2, 19]. Biopsy of lesions in the small bones of a hand or foot may be more easily accomplished under real-time fluoroscopic guidance, which allows more flexibility for positioning the affected area.
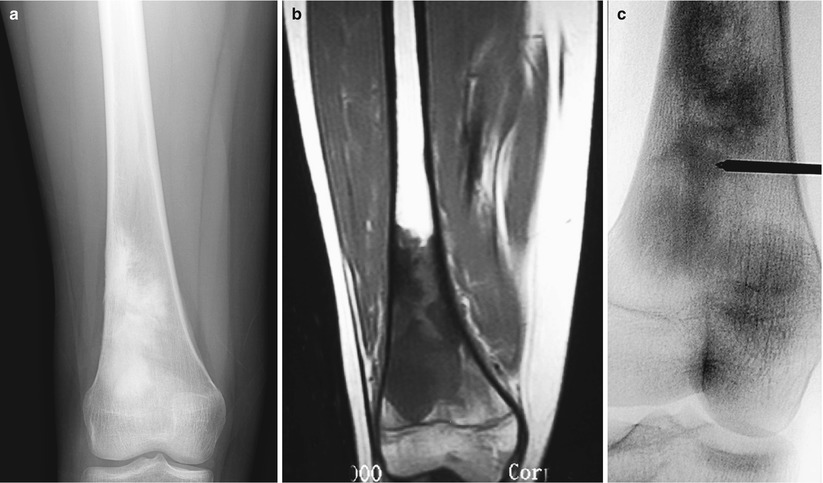
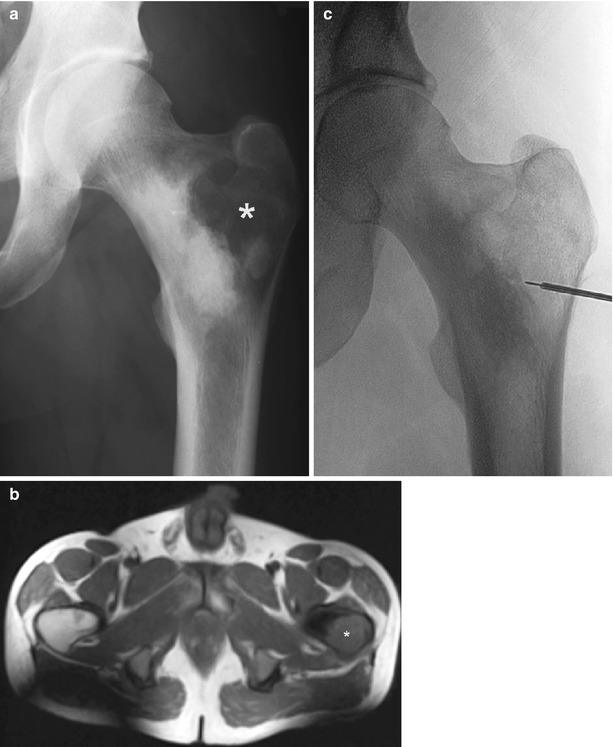

Fig. 6.2
Fluoroscopic-guided biopsy of sclerotic bone lesion. A 16-year-old girl complained of pain in the right distal thigh. AP radiograph of the right femur (a) showed a dense sclerotic lesion. Coronal T1-weighted image of the femur (b) showed abnormal signal in the distal femur with no associated soft-tissue mass. Biopsy of the distal femur was performed under fluoroscopic guidance (c). Pathology showed osteosarcoma

Fig. 6.3
Fluoroscopic-guided biopsy of lytic bone lesion. A-19-year-old man presented with pain involving the left hip. AP radiograph of the left femur (a) shows a lytic lesion involving the greater trochanter of the left femur with sclerotic border extending medially. Axial T1-weighted image of the pelvis (b) confirms mixed lytic-sclerotic bone lesion involving the proximal left femur. The lytic lesion in the greater trochanter was sampled under fluoroscopic guidance (c). Pathology showed osteosarcoma
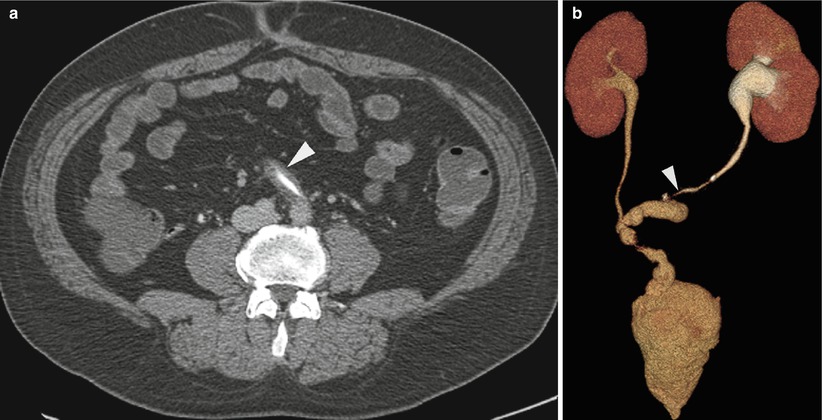
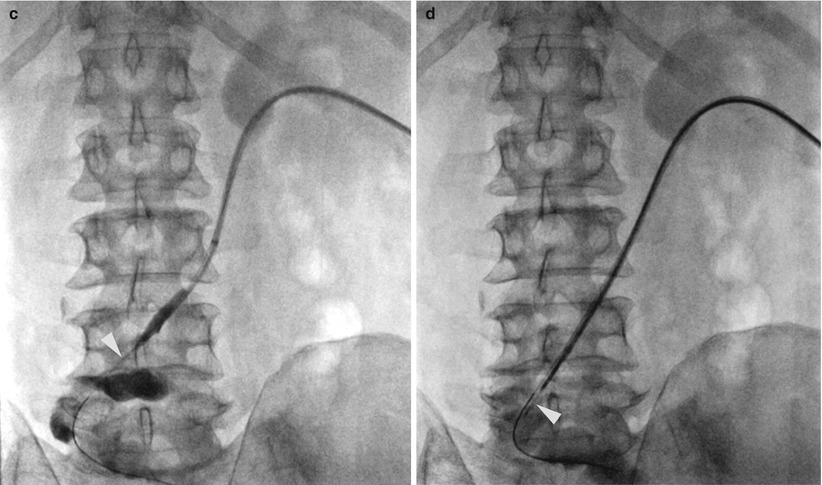
Endoluminal brush or forceps biopsy of strictures within the bile ducts or ureters (Fig. 6.4) requires fluoroscopic examination of the biliary or urinary tract [5, 6]. Finally, transjugular liver or kidney biopsy requires fluoroscopy to identify and select the appropriate veins (Fig. 6.5) [7, 8]. Endoluminal or transvenous biopsy devices are manipulated under real-time fluoroscopy and positioned appropriately to optimize the biopsy yield.