Fig. 11.1
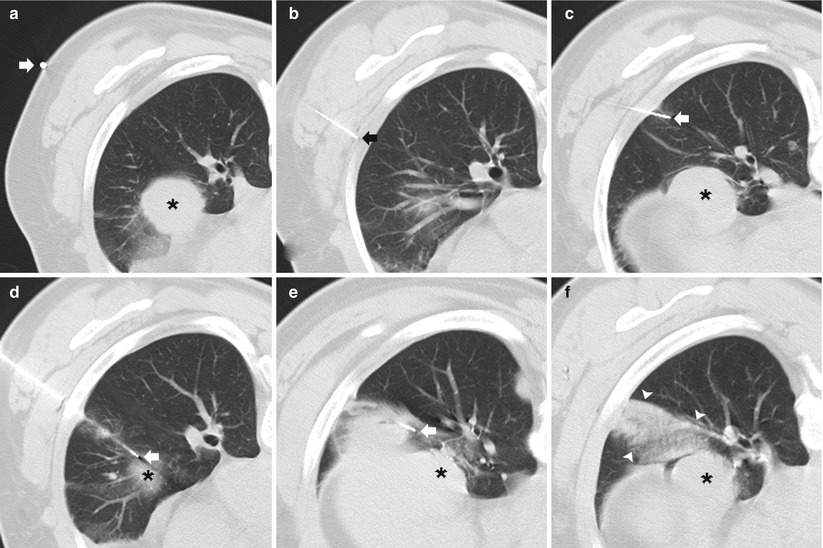
Solitary pulmonary nodule: two different patients (a–b and c–d) undergoing TCNB for an indeterminate pulmonary nodule. (a) Pre-biopsy image demonstrates a 20 mm lobulated nodule within the left lower lobe in close proximity to the oblique fissure with surrounding centrilobular emphysema. (b) A 19-gauge coaxial needle is placed into the nodule (arrow). Biopsy results demonstrated poorly differentiated adenocarcinoma. (c) Pre-biopsy CT image demonstrates a 13 mm lobulated nodule within the left upper lobe with surrounding centrilobular emphysema. (d) A 19-gauge coaxial needle is placed into the nodule (arrow). Biopsy results demonstrated a hamartoma
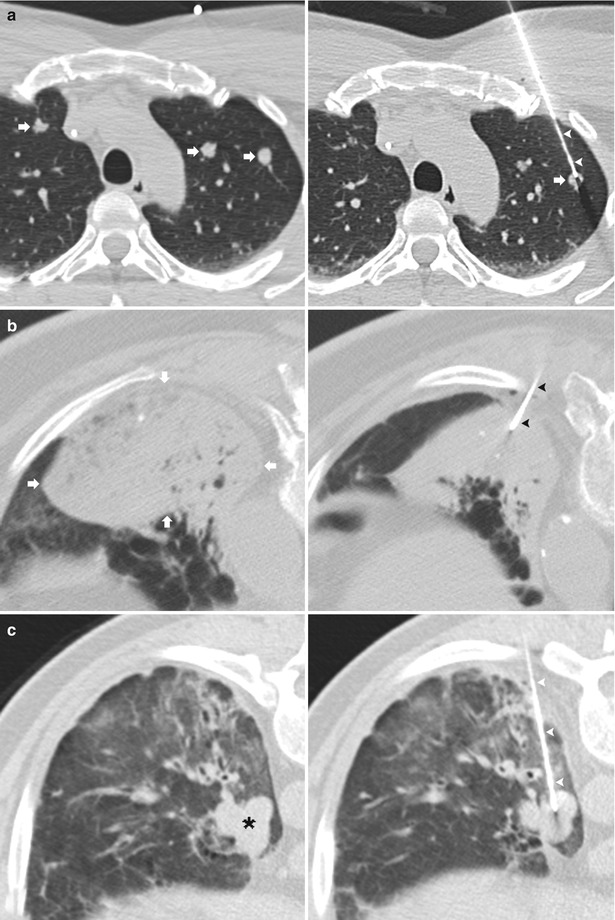
Fig. 11.2
Biopsy indications: three different patients undergoing TCNB. (a) Pre-biopsy CT image demonstrates multiple pulmonary nodules (arrows). A 19-gauge coaxial needle (arrowheads) is positioned in the lateral left upper lobe nodule (arrow) via a parasternal approach. Biopsy results demonstrated colorectal adenocarcinoma. (b) Pre-biopsy CT image demonstrates a persistent left lower consolidation (arrows). A 19-gauge coaxial needle (arrowheads) is positioned in the consolidation via a posterior approach. Biopsy results demonstrated bronchoalveolar carcinoma. (c) Pre-biopsy CT image demonstrates a lobulated left lower lobe nodule in a patient with Hodgkin’s lymphoma. A 19-gauge coaxial needle (arrowheads) is positioned in the nodule via a posterior paraspinal approach. Biopsy results demonstrated treated lymphoproliferative disease
An increasingly common indication for TNB, particularly in the immunocompromised population, is a focal area of consolidation or suspected lung abscess in which bronchoscopy, or sputum cytology and culture have not yielded a diagnosis [9, 10]. Additional indications for TNB include enlarging ground glass opacities (Fig. 11.3) [11, 12] and evaluating for local recurrence following therapy (Fig. 11.4) [7].
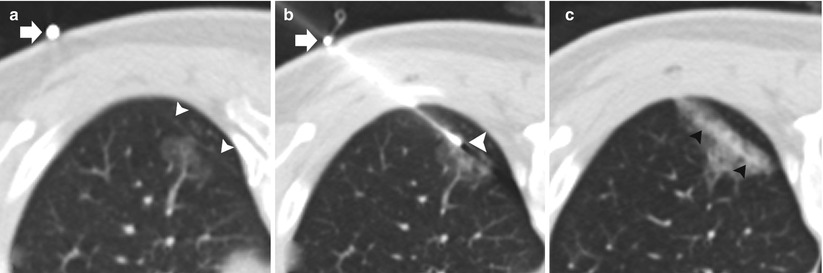
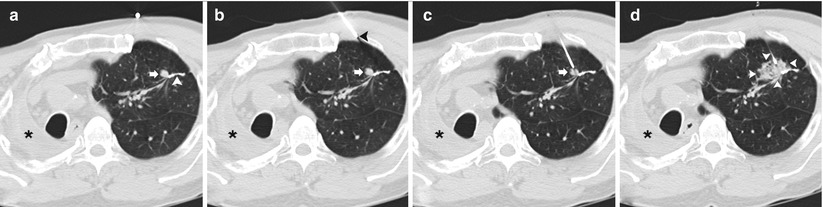

Fig. 11.3
Ground glass nodule. (a) Pre-biopsy CT image demonstrates a 23 mm ground glass nodule within the left lower lobe in close proximity to the oblique fissure (arrowheads). A radiopaque marker (arrow) is placed on the skin indicating a posterolateral approach to avoid transgression of the oblique fissure (arrowheads). (b) A 19-gauge coaxial needle is advanced via a perifissural route with confirmation of the needle tip (arrowhead) within the target lesion. Prior to obtaining specimens, the attachable depth marker (arrow) is positioned to prevent inadvertent advancement of the coaxial needle. (c) Post-biopsy images demonstrate a small amount of perinodular hemorrhage and autologous blood along the parenchymal needle tract (arrowheads). Biopsy results demonstrated bronchoalveolar cell carcinoma

Fig. 11.4
Suture recurrence: 66-year-old man status post right pneumonectomy for primary bronchogenic adenocarcinoma and left upper lobe wedge resection of a metachronous primary bronchogenic carcinoma. (a) Pre-biopsy CT image demonstrates postoperative changes of a right pneumonectomy and an 8 mm nodule (arrow) within the left upper lobe adjacent to the microsuture line (arrowhead). (b) A 19-gauge coaxial needle is placed in the left anterior chest wall with the tip in the extrapleural space (arrowhead) in the expected path to the target lesion (arrow). (c) The 19-gauge coaxial needle is advanced into the lung with the tip adjacent to the nodule (arrow). (d) Following needle tip confirmation, multiple 20-gauge core needle samples were obtained with development of perinodule hemorrhage (arrowheads). Biopsy results demonstrated recurrent adenocarcinoma
The position of the lesion and its appearance on computed tomography (CT) continue to determine whether bronchoscopic biopsy or TNB is the more appropriate technique for establishing a diagnosis. In general, large central lesions with an endobronchial component are likely to be accurately diagnosed with flexible bronchoscopy, whereas peripheral tumors, especially those smaller than 3 cm in diameter, without a bronchus entering the proximal portion of the lesion are best suited for TNB [5, 7]. With improvements in technology and technique, TNB is an accurate method of diagnosing lesions regardless of their size or location where nodules less than 1 cm in diameter can be safely and accurately sampled [13]. This then provides new diagnostic options for patient populations requiring evaluation of an indeterminate nodule including patients undergoing staging and transplant evaluation (Fig. 11.5).
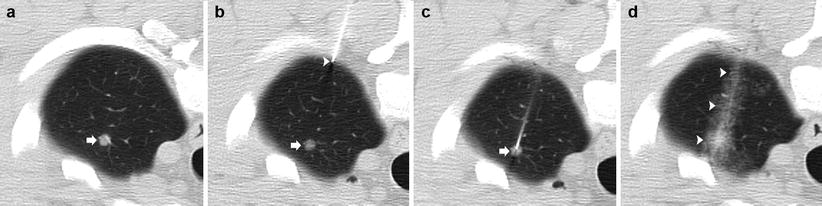

Fig. 11.5
4 mm nodule: 50-year-old man with hepatocellular carcinoma undergoing transplant evaluation with an indeterminate solitary pulmonary nodule. (a) Pre-biopsy CT image demonstrates a 4 mm nodule within the left upper lobe (arrow). (b) A 19-gauge coaxial needle is placed in the left anterior chest wall with the tip in the extrapleural space (arrowhead) in the expected path to the target lesion (arrow). (c) The 19-gauge coaxial needle is advanced into the lung with confirmation of the tip within the nodule (arrow). (d) Post-biopsy images demonstrate a small amount of perinodular hemorrhage and autologous blood along the parenchymal needle tract (arrowheads). Biopsy results demonstrated a necrotizing granuloma
Regardless of the indication, the guiding principle used in all cases of TNB is that the potential benefits of a tissue diagnosis will affect patient management in such a manner that outweighs the risks of the procedure [5, 6, 14].
Contraindications
Although some authors claim that any case of an anatomic or functional single lung or severe pulmonary arterial hypertension (>40 mmHg) are absolute contraindications to TNB [2, 5], others insist that there are no absolute contraindications to the procedure [7]. Overall, there are few relative contraindications to TNB, most authors agreeing that several preexisting conditions significantly increase the complication rate of TNB or render any complication more significant and therefore constitute relative contraindications to TNB. Relative contraindications include the inability to cooperate (lie flat and breath hold), although rectified with general anesthesia and deep sedation; coagulation disorders (international normalized ratio >1.3 or platelets <50,000) and anticoagulation medications; bulla or severe emphysema; relatively prominent airways or vasculature within the anticipated biopsy path; cardiac insufficiency; and pneumonectomy, although some authors believe that TNB can be safely performed even in this population if the proper preparations are made for rapid chest tube insertion (Fig. 11.4) [15]. Above all, TNB should be limited to cases that are truly indicated, technically feasible, and for which the anticipated benefits outweigh the risks.
Technique
Approach and Relevant Anatomy
Multiple anatomic factors should be taken into account when developing an approach for a TNB. The site of entry, adequate pleural anesthesia, and awareness of the bronchovascular planes, the fissures, and the hilar and mediastinal structures are critical in maximizing patient comfort to potentiate the success of the biopsy while minimizing the risk for significant complications.
Initial adequate placement of the coaxial needle is essential for achieving pleural anesthesia and optimal trajectory for needle placement into the target lesion. Adequate pleural anesthesia is the cornerstone of a successful biopsy, as patient comfort and cooperation are of the utmost importance. The parietal pleural is the second most sensitive structure aside from the skin in the path of the needle. The costal and diaphragmatic parietal pleura are innervated by the overlying intercostal nerve, where inadequate anesthesia is characterized by significant pain and discomfort along the dermatome or referred pain to the ipsilateral shoulder. Effective anesthesia of the parietal pleura requires the precise placement of the anesthesia needle tip in an extrapleural location immediately adjacent to and superficial to the parietal pleura (Fig. 11.6), while avoiding both the underlying parietal and visceral pleura, which if violated increases the risk of an early pneumothorax. Proper needle tip placement requires the combination of exact measurements obtained from localization scans and operator needle control. As the needle penetrates the different layers of the chest wall, the operator will feel many “pops,” representing the needle traversing the different chest wall structures, the most important of which is the endothoracic fascia as its passages signify the exact location of the extrapleural fat space immediately superficial to the parietal pleura, where a few millimeters can mean the difference between a straightforward biopsy and one complicated by an early pneumothorax (Fig. 11.7).
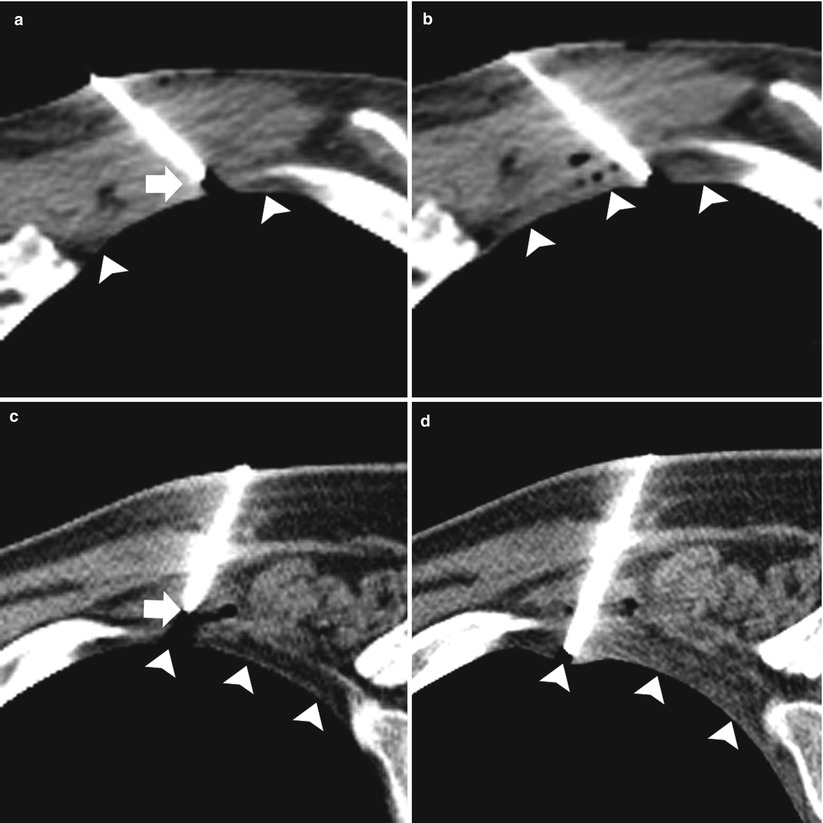
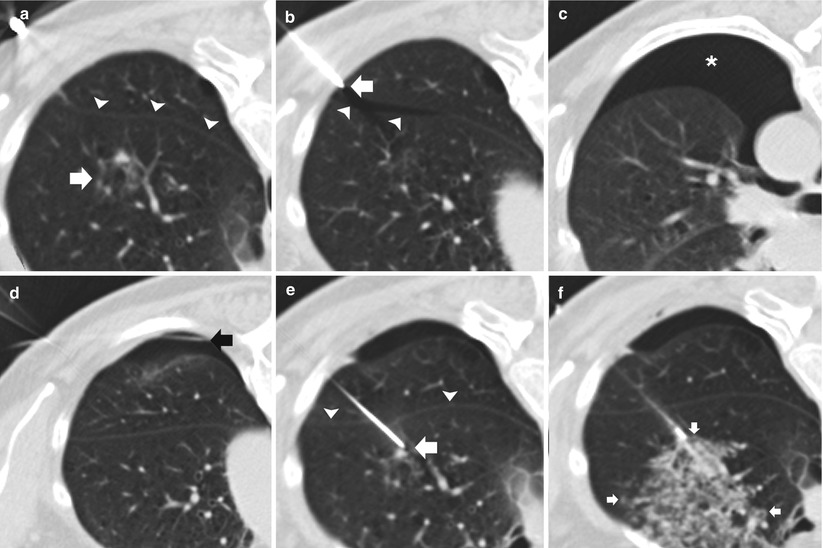

Fig. 11.6
Pleural anesthesia: two different patients (a–b and c–d) undergoing TCNB. (a and c) Preanesthesia: 19-gauge coaxial needle with the tip (arrows) superficial to the extrapleural fat (arrowheads). (b and d). Postanesthesia: 19-gauge coaxial needle has been advanced with the tip in the extrapleural fat status post administration of 1 % lidocaine in the extrapleural space (arrowheads)

Fig. 11.7
Early pneumothorax. (a) Pre-biopsy CT image demonstrates an 8 mm ground glass nodule (arrow) within the left upper lobe in close proximity to the oblique fissure (arrowheads). (b) Following placement of a 19-gauge coaxial needle, the tip (arrow) is seen 3 mm into the thoracic cavity resulting in a premature pneumothorax with air tracking into the oblique fissure (arrowheads). (c) Repeat imaging demonstrates an enlarging pneumothorax consistent with a persistent air leak. (d) Interval placement of a small angiocatheter (black arrow) with interval decrease in size of the pneumothorax status post aspiration. (e) A second 19-gauge coaxial needle is advanced into the target lesion via a transfissural route (arrowheads) to complete the procedure despite the air leak. (f) Following needle tip confirmation within the nodule, multiple 20-gauge core needle samples were obtained with development of perinodule hemorrhage (arrows). Biopsy results demonstrated invasive adenocarcinoma with bronchoalveolar cell features
An understanding of the central bronchovascular computed tomography (CT) anatomy allows the development of a safe transthoracic route to a central lesion. The pulmonary hila are composed of the major bronchi with accompanying pulmonary arteries and pulmonary veins that extend radially into the lobes, segments, and subsegments of the lungs. It is often difficult to avoid the vessels and airways in the periphery of the lung, but fortunately, such disturbances result in minimal to no significant complications. For central lesions, when developing a percutaneous transthoracic route, the radial nature of the bronchovascular structures from the hila to the periphery of the lung typically provides an abronchovascular route to the central portions of the lung and thus allowing safe access to a centrally located lesion (Fig. 11.8). By exploiting this abronchovascular plane, the risk of a threatening pulmonary hemorrhage with or without hemoptysis, and in a worst-case scenario, air embolism can be significantly reduced. In addition to conventional axial images, sagittal and coronal reformations can better delineate the relationship of the target lesion with the surrounding bronchovascular structures and may be beneficial in developing a safe transthoracic route. Of particular interest is the pulmonary venous anatomy as transgression should be avoided at all costs to minimize the risk of hemorrhage, bronchovenous fistula, and air embolism.
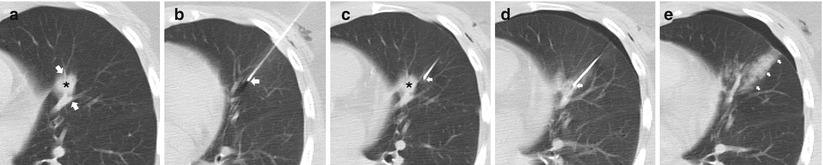

Fig. 11.8
Abronchovascular route. (a) Pre-biopsy CT image demonstrates a spiculated 15 mm nodule within the lingula intimately associated with the pulmonary vasculature (arrows). (b) A 19-gauge coaxial needle (arrow) is carefully advanced towards the target lesion. (c) Utilizing the abronchovascular plane, the needle (arrow) is slowly advanced into the centrally located target lesion . (d) Following needle tip confirmation, multiple 20-gauge core needle samples were carefully obtained with development of perinodule hemorrhage. (e) Upon removal of the coaxial needle, the patient’s blood is injected into the needle tract (arrows) with a small but stable pneumothorax. Biopsy results demonstrated invasive adenocarcinoma with bronchoalveolar cell features
Awareness of the interlobar and accessory fissures is important, as these fissures should be avoided to reduce the risk of a pneumothorax, which may result in a problematic or persistent air leak. The relevant fissures are comprised of the major, minor, and accessory fissures. These fissures are best visualized on thin, less than 3 mm, CT sections; they appear as a white line that typically can be avoided when developing a path to the target lesion. However, if thin sections are not available, fissural position can be inferred from the location of a 1–2 cm thick avascular plane of lung. The minor fissure is usually difficult to visualize, as it parallels the plane of the scan, but it can be readily visualized on coronal and sagittal reconstructions. The fissures are not always complete and are often partial. Aziz et al. [16] catalogued in a large study population the incidence of partial or absent fissures and found centrally absent right major, left major, and right minor fissures in 48 %, 43 %, and 63 % of patients, respectively. Once recognized, incomplete fissure anatomic variation can be taken advantage of while planning a translobar approach to a central lung target (Fig. 11.9). Accessory fissures, seen in up to 30 % of individuals [17], should also be considered while planning an approach; common accessory fissures include the inferior accessory fissure that separates the medial basal segment from the remaining basal segments, the azygos fissure (composed of four layers of pleura: two visceral and two parietal) that represents an invagination of the right apical pleura by the azygos vein, the superior accessory fissure that separates the superior segment from the basal segments of the lower lobe, and the left minor fissure that separates the lingula from the remaining portions of the upper lobe.
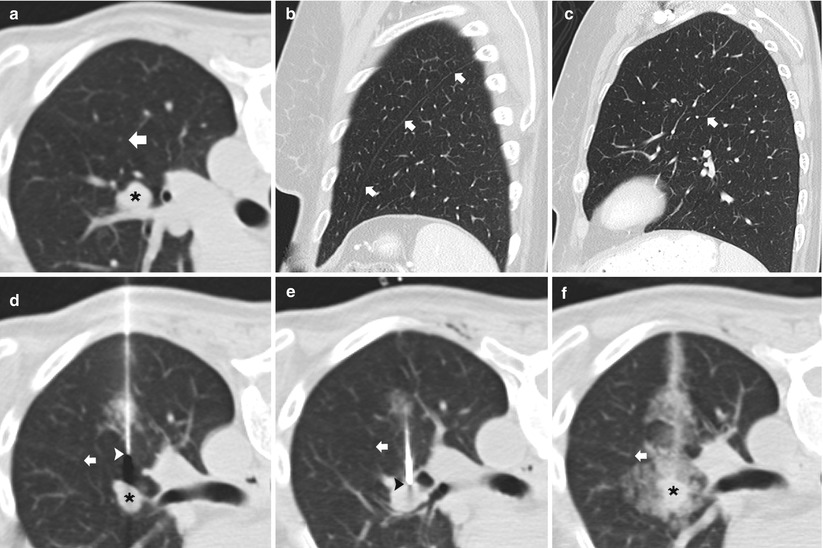

Fig. 11.9
Incomplete fissure. (a) Baseline axial CT image shows a centrally located 15 mm nodule in the left upper lobe with an incomplete oblique fissure (arrow) seen posterior and lateral to the pulmonary nodule. (b) Sagittal multiplanar reformats demonstrates a complete left oblique fissure (arrows) laterally. (c) Sagittal multiplanar reformats medial to that in (b) confirms that the left oblique fissure is incomplete (arrow) medially. (d) and (e) The incomplete fissure is again seen (arrows) allowing the 19-gauge coaxial needle to approach the left upper lobe nodule via the preferred posterior approach through the left lower lobe. (f) Following needle tip confirmation within the nodule, multiple 20-gauge core needle samples were carefully obtained with development of mild hemorrhage surrounding the nodule . Biopsy results demonstrated metastatic melanoma within an intrapulmonary lymph node
When planning a percutaneous transthoracic needle biopsy, lesions in a paramediastinal location must be approached and biopsied with caution, as they pose significant risk of injury to the cardiomediastinal structures. Those structures where the needle should preferably have a parallel course include the heart, the aorta and its branches and superior and inferior vena cava (Fig. 11.10) [2].
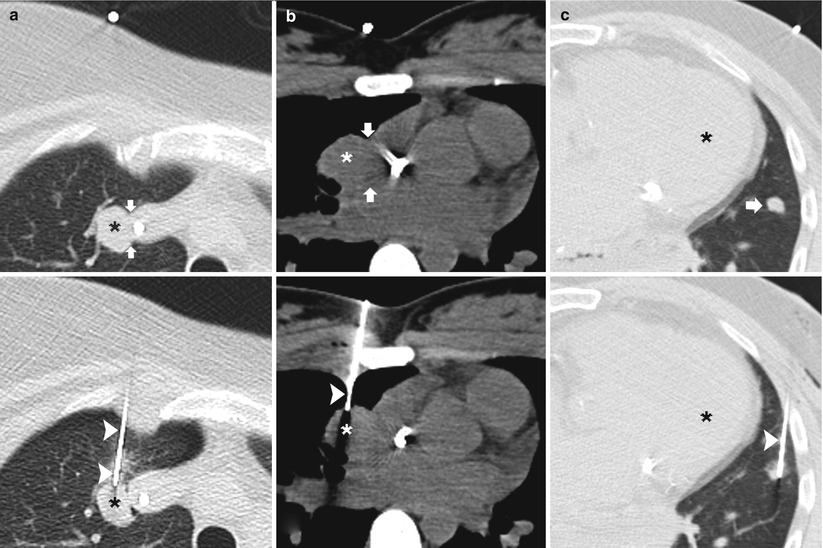

Fig. 11.10
Parallel approach: three different patients undergoing TCNB. (a) Pre-biopsy CT image demonstrates a right upper lobe nodule adjacent to the superior vena cava (arrows). A 19-gauge coaxial needle (arrowheads) is advanced into the lung parallel to the mediastinum with the tip in the target lesion . Biopsy results demonstrated metastatic synovial sarcoma. (b) Pre-biopsy CT image demonstrates a right upper lobe nodule adjacent to the right atrium (arrows). A 19-gauge coaxial needle (arrowhead) is advanced into the lung parallel to the heart with the tip in the target lesion . Biopsy results demonstrated lymphoma. (c) Pre-biopsy CT image demonstrates a lingula nodule (arrow) in close proximity to the left ventricle . A 19-gauge coaxial needle (arrowhead) is advanced into the lung parallel to the heart with the tip in the nodule. Biopsy results demonstrated dense fibrous tissue with chronic lymphoplasmacytic inflammation
Preprocedure Evaluation
Evaluation of a potential candidate for TNB is preferably performed in an outpatient setting. Directed patient history and physical examination are necessary, with special attention given to the patient’s cardiopulmonary status and ability to cooperate, including the ability to perform adequate breath hold and the ability to lie still for an extended period of time. A thorough evaluation of the patient’s medication history is important, with attention paid to anticoagulant and antiplatelet medications, as these need to be discontinued prior to the procedure. Patients on warfarin require conversion to subcutaneous or low molecular weight heparin, which is then stopped at least 24 h before the procedure. Aspirin and clopidogrel bisulfate should be discontinued for at least 7 days. Increasingly, patients may require biopsy while on or immediately after non-chemotherapeutic systemic therapy, such as bevacizumab, and in these situations, additional precautions should be undertaken. Review of a recent electrocardiogram (ECG) and pulmonary function tests (PFTs) is particularly important in patients with comorbid heart and/or lung disease or prior resection. Many patients undergoing TNB are or have been smokers, and PFTs may be obtained to establish adequacy of oxygenation, pulmonary reserve, and flow-volume spirometry. Recent CT, magnetic resonance (MR), and computed tomography-positron emission tomography (CT-PET) scans should be available to ensure amenability of biopsy and to preliminary map out a safe needle path. In addition, the preprocedure CT demonstrates comorbid disease in the lung and the location and relationship of the lesion to vital structures, emphysema, and bulla.
Day of the Procedure
An informed consent for the procedure and other specific interventions should be obtained, detailing the benefits, risks, and alternatives. Laboratory data, including prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), and on minor occasion, bleeding time (BT) are required the day of the procedure or at least within the past 7 days, especially if the patient is on anticoagulation. A complete blood count (CBC), including hemoglobin and hematocrit, white count, and, most importantly, platelets, is recommended. Creatinine should be obtained if the administration of iodinated contrast is required to delineate vascular structures and non-necrotic tumor margins. Every patient should have adequate peripheral intravenous access.
Breathing Instructions
Different techniques are used with regard to patient breath holding during the procedure and needle insertion, specifically consistent or constant volume breath holds versus continuous shallow breathing. When performing a TNB in a fully cooperative patient, constant volume breath holds allow for a relatively predictable nodule position resulting in shorter procedure times. The patient is instructed to take a small and comfortable breath that can be reproduced and held for 10–15 s. This is particularly helpful when biopsying small nodules near the diaphragm, cardiomediastinal structures, lingula, and right middle lobe, where any degree of respiratory motion can result in a significant change in the relative position of the needle, lesion, and surrounding vital nontarget structures [1, 2, 18].
Alternatively, for those patients who cannot hold their breath in a consistent fashion, allowing them to perform continuous shallow breathing takes the unpredictable changes in lung volume out of the equation. This requires an understanding of the nodule, needle, and entry site relationship throughout the respiratory cycle based on available imaging (fluoroscopy, CT, and CT fluoroscopy). Depending on the phase of the respiratory cycle, adjustments are made based on the relative position of the nodule and the needle, and additional adjustments are made accordingly as the patient is breathing [1, 18].
In addition, breathing instruction can be altered during the procedure to aid in nodule localization. Understanding the effect that breathing has on a nodule based on the volume can aid in instructing the patient to take a shallower or deeper breath depending on the relative needle and lesion position. As a general rule, inspiration resulting in deeper volumes has the effect of moving the nodule in a caudal direction, and expiration resulting in shallower volumes has the effect of moving the nodule in a cranial direction. Thus, if attempts have failed to steer the needle to the nodule, intra-procedural breathing instructions may be altered to bring the nodule in line with the needle. Note is made that a lesion closer to the diaphragm demonstrates a greater degree of movement with variation in lung volume [1].
Sedation
Transthoracic needle biopsy is performed primarily as an outpatient procedure with preferably no or minimal conscious sedation as the patient is required to maintain an appropriate level of consciousness for cooperation and monitoring. If conscious sedation is required, a typical conscious sedation protocol includes midazolam and/or morphine sulfate titrated slowly to achieve the appropriate level of comfort. In addition, the antitussive effect of morphine is an added benefit to help decrease the incidence of significant coughing episodes. In certain circumstances, operators have used deep conscious sedation and even general anesthesia [19], in patients who are unable to lay still, breath hold, and in children. The advantages of deep conscious sedation and general anesthesia include a controlled patient environment, greater degree of intra-procedural patient comfort, better control of the airway, and the presence of an expert in cardiopulmonary management in case of a complication. The disadvantages of general anesthesia include higher cost, logistical challenges requiring a second participating service, longer procedure times related to anesthesia setup and availability, higher potential for pneumothorax due to positive pressure ventilation, and the risks specific to deep sedation and general anesthesia.
Imaging
Percutaneous TNB can be performed under fluoroscopy, CT, CT fluoroscopy, or ultrasonography; the choice of image guidance modality is dependent upon the size and location of the lesion, the availability of imaging systems, and operator expertise. Localization of the lesion by chest radiography and CT is necessary to determine the method of biopsy. The depth of the lesion and its relation to the major airways and vasculature, fissures, mediastinum, and ribs are used to plan the biopsy route.
Traditionally, percutaneous TNB was performed under fluoroscopic guidance. This technique has the advantages of real-time visualization, short procedure duration, and low cost. However, because of its limited ability to localize small lesions, to guide access to central lesions, and to avoid bullae and vascular structures in the needle track, fluoroscopy is now reserved for large, peripheral lesions [5, 6].
In most centers, CT is now the standard imaging modality for TNB. CT enables selection of a biopsy route with the shortest distance between pleura and lesion and allows access to central lesions, while avoiding airways and vessels, fissures, bullae, and pronounced emphysema (Fig. 11.11). In addition, CT aids in distinguishing necrotic from solid portions of the lesions and allows unequivocal documentation of the needle tip within the lesion (paramount in the interpretation of absence of malignant cells) [20]. Procedure duration, one of the most commonly cited drawbacks of CT guidance, has significantly decreased with the advent of CT fluoroscopy [21]. This is of particular importance in less cooperative patients. In addition, CT fluoroscopy offers the advantage of near real-time visualization of needle placement. Real-time imaging is important within the lung, where extensive respiratory movement is present, causing small target lesions to shift and even disappear from the scan plane during needle advancement. Lack of real-time imaging has been cited as one of the most considerable reasons why CT-guided biopsies are unsuccessful or need to be repeated [22]. CT fluoroscopy has been shown to facilitate biopsy of smaller lesions as well as lesions situated in less favorable locations such as those in the costophrenic recess or close to the mediastinum. One of the major concerns regarding CT fluoroscopy remains the increased operator-related radiation exposure (although patient-related radiation exposure is essentially unchanged) [21].
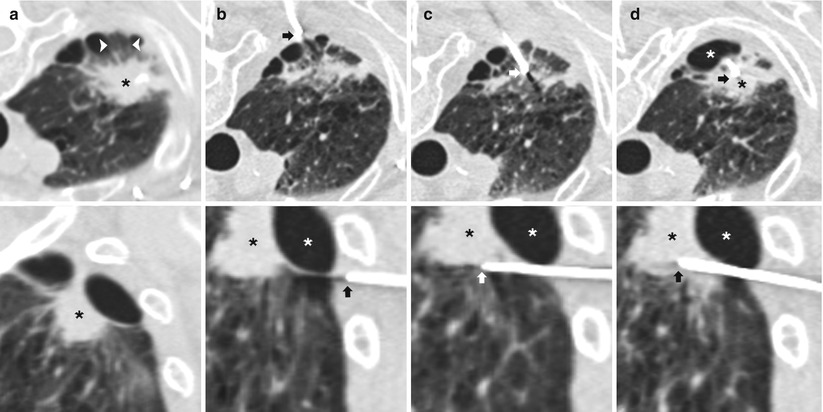

Fig. 11.11
Multiplanar reformats. (a) Axial (top) and sagittal (bottom) CT images demonstrates an irregular right apical nodule (black *) with surrounding emphysema and peripheral bullae. An intact secondary pulmonary lobule (arrowheads) is seen peripheral to the nodule and surrounded by bullae. (b) Axial (top) image demonstrates placement of a 19-gauge coaxial needle in the posterior chest wall with the tip in the extrapleural space (arrow) in the expected path to the target lesion. Sagittal (bottom) reformat images demonstrates the exact relationship of the target nodule (black *), bulla (white *), and needle tip (arrow). (c) Axial (top) image demonstrates advancement of 19-gauge coaxial needle into the lung via the intact secondary pulmonary lobule. The sagittal (bottom) reformat image demonstrates the needle tip (arrow) inferior to the bulla (white *) and target lesion (black *). (d) Axial (top) image demonstrates repositioning of the 19-gauge coaxial needle into the target lesion (black *). The sagittal (bottom) reformat image confirms needle tip (arrow) placement into the nodule (black *) while avoiding the bulla (white *). Biopsy results demonstrated a fibroelastic scar with apical fibrous cap
In some instances, TNB can be performed under ultrasound guidance. Major advantages of ultrasound include real-time visualization of needle placement, portability, ability to target nonvascular, non-necrotic portions of a mass for sampling, and lack of exposure to ionizing radiation. Like CT, ultrasound also increases the likelihood of a positive biopsy in cavitating lesions. Ultrasound-guided TNB is limited to regions that provide an adequate acoustic window to the lesion and is best suited for peripheral pulmonary or mediastinal masses that abut the pleura (Fig. 11.12) [5, 7].
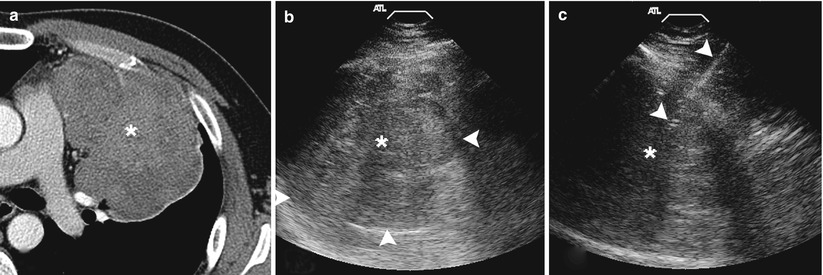

Fig. 11.12
Ultrasound biopsy. (a) Contrast-enhanced axial CT image demonstrates a large enhancing mass within the left hemithorax abutting the chest wall providing an adequate acoustic window. (b) Grey-scale ultrasound image demonstrates a heterogenous predominantly hypoechoic mass with the lung-mass interface clearly delineated (arrowheads). (c) Utilizing ultrasound guidance, a 19-gauge coaxial needle (arrowheads) is advanced into the mass . Biopsy results demonstrated a poorly differentiated carcinoma
Devices
A variety of needle sizes, tip designs, and sampling mechanisms are used in TNB. Ideally, a biopsy needle should maximize specimen yield while minimizing bleeding complications. Bleeding complications are avoided today by using needles smaller than 18 gauge. Both single- and multiple-pass coaxial needles have been used in percutaneous TNB. The coaxial technique, which involves inserting a thin inner needle through a larger outer needle positioned within the lesion, has several advantages over the single-needle technique, including limitation of the number of pleural punctures, easy repositioning of the needle, and the possible use of techniques to prevent leakage of air [18].
The biopsy needles currently used in TNB can be divided into two broad categories: aspirating and cutting needles [7]. Commonly used aspirating needles include the Chiba, spinal, Westcott, and Greene needles, most ranging in size from 20 to 22 gauge [6]. Aspirating needles, when accurately placed within the lesion of interest, can provide high-quality cellular material for the diagnosis of malignancy [7]. When TNB is used to isolate causative organisms in pneumonia or lung abscess, a 22-gauge Chiba or spinal needle usually provides an adequate specimen [7]. Cutting or core needles are generally larger than aspirating needles (usually 18–20 gauge) and are designed to provide histologic material using a circumferential cutting tip (e.g., Greene or Turner needles), a receptacle slot proximal to the tip (e.g., Westcott needle), an automated spring-loaded end cut or side-notched device (e.g., ASAP or Temno, Angiotech needles), or a drill bit stylet contained within a guiding cannula [7]. Core biopsy is best suited for large (>3 cm) peripheral lesions that abut the pleura; however, its use in the diagnosis of smaller (<3 cm) lesions has been reported [23] and increasingly more common. Coaxial models with small diameters have become available, allowing multiple core specimens to be obtained with a single pleural puncture [24]. Debate exists in the literature as to whether cutting needles offer any advantage over fine-needle aspiration in the diagnosis of malignancy [25, 26], but with the advent of molecular profiling, the importance in obtaining core samples is well established [1–4]. In addition, cutting needles are felt to be more accurate in the specific diagnosis of benign lesions, lymphoma, and in the setting where expert cytopathology is unavailable [24, 27, 28]. The two techniques are often used in complementary fashion, with cutting needles proposed when the diagnosis of malignancy is not made with fine-needle aspiration [29]. The initial choice between fine-needle aspiration and core needle biopsy depends on several factors, including operator expertise, the risk of complication, and the availability of an on-site cytopathologist. The use of core biopsy is now preferred in the current era of molecular profiling, in the absence of an on-site pathologist, and when the diagnosis of benign lesion or lymphoma is suspected [1–4, 24, 27, 28].
Biopsy Technique
The goal in performing TNB is to obtain sufficient material for diagnosis and characterization while minimizing potential complications. Special techniques may be utilized in TNB to maximize diagnostic yield and to avoid and/or minimize significant complications. These techniques are especially important when performing TNB in high-risk patients with significant cardiopulmonary disease as the potential impact of having to repeat a false-negative biopsy or experiencing a complication in these patients is much greater [15]. The following section outlines the biopsy process while focusing on various techniques that have been described to maximize success and to minimize potential complications. These include patient positioning, needle advancement, enhanced targeting, and procurement and evaluation of the specimen.
Patient positioning on the CT table warrants special attention as proper positioning is crucial for maximizing patient comfort and procedural success while minimizing potential complications. In general, the patient should be positioned so that the skin entry site allows the shortest, most vertical path to the lesion while avoiding fissures, bullae, major airways, and prominent vasculature. The shortest distance from skin to the tumor is optimal, although a lateral approach is less favorable due to the increased and opposite motion of ribs to the lungs during breathing, making needle placement difficult while increasing the risk of an air leak (Fig. 11.13). When equivocal, a posterior approach with the patient in the prone position is preferable to an anterior approach with the patient in the supine position, because the posterior ribs are less subject to respiratory variation and maintain a more consistent intercostal window with less need to rely upon breathing techniques (Fig. 11.14) [30]. In addition, since it is preferable for the patient to lie with the biopsy site dependent after completion of the procedure, lying supine post-procedure for an extended period of time is generally easier for the patient. If a supine position is required, an anterior parasternal approach is the next preferred route.