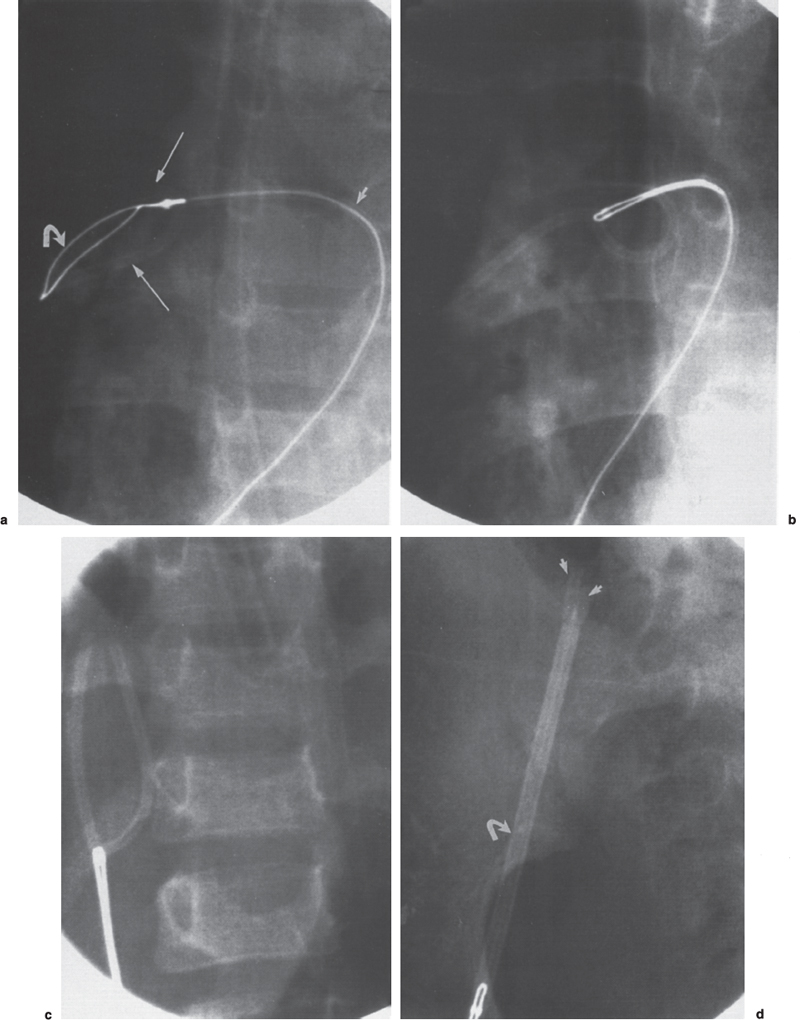
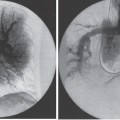
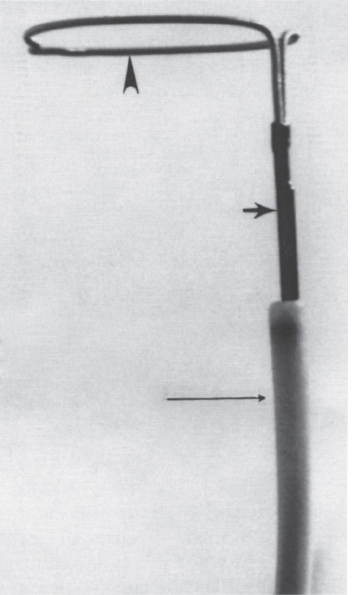
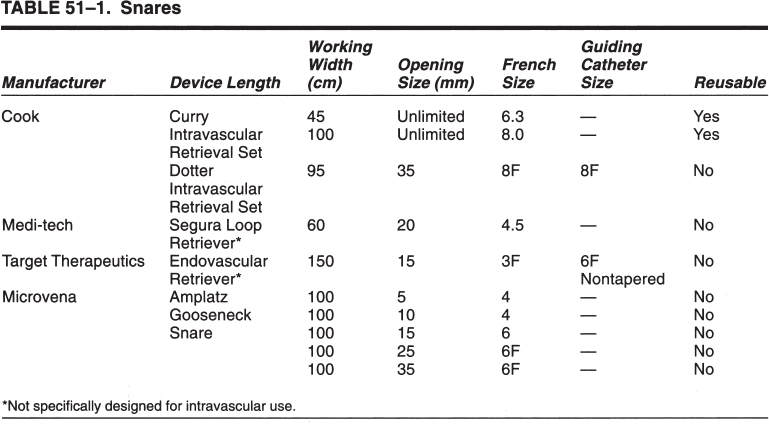
Foreign Body Retrieval Since Turner and Sommers reported the first nonsurgical retrieval of an embolized intravascular foreign body in 1954,1 hundreds of case reports have appeared in the literature describing various techniques that can be used for foreign body retrieval. The most common iatrogenic foreign bodies are broken through-the-needle polyethylene and diagnostic catheter fragments.2–5 Other common objects include guidewires and guidewire fragments (Fig. 51–1 ),6,7 intravascular embolization coils,8–10 bullets,3,11,12 inferior vena cava filters,13–16 intravascular metallic stents,17–19 and vascular sheaths.20,21 Embolized foreign bodies most commonly come to rest within the superior and inferior vena cava, right side of the heart, and pulmonary circulation.3–5 Some reports suggested that the risk of removal of a foreign body, such as an inferior vena cava filter from the heart, may be riskier than conservative management.22 It is generally accepted, however, that intracardiac foreign bodies, even a relatively soft catheter fragment, possess a significant risk to the patient because of the potential for (1) repeated (septic) pulmonary embolism, (2) sepsis, (3) endocarditis, (4) cardiac perforation with hemopericardium, and (5) cardiac arrhythmias.1,7,23,24 In an extensive literature review by Fisher and Ferreyro,23 71% of 220 cases of retained intravascular (catheter fragment) foreign bodies directly resulted in serious morbidity or death resulting from the aforementioned risks. In a review of 11 cases by Taylor and Rutherford,25 five of seven patients with intracardiac catheter fragments managed conservatively died of sepsis. Brown and Kent26 Johnson27 each reported a case of cardiac perforation and death secondary to embolized catheter fragments. Numerous devices, including catheters, snares, stone-retrieval baskets, forceps, and combinations of these devices, have been used for successful percutaneous retrieval of embolized foreign bodies from both the central and peripheral venous circulation. Percutaneous retrieval usually can be performed in a relatively short time in even the sickest patients and almost always without complications. Before the development of percutaneous methods of foreign body retrieval, this group of patients would require surgical foreign body retrieval, a procedure that often carries a significant morbidity and mortality. This chapter discusses the use of these devices for intravascular foreign body retrieval. Loop-snare Technique This technique was originally described by Massumi and Ross in 196728 for removal of a transvenous pacing catheter from the right side of the heart. The device was constructed from a 135-cm guidewire looped within a 60-cm 12F catheter. One year later, Harnmenneister and Kennedy29 modified the loop-snare device by using a smaller 0.025-inch guidewire and a 40-cm 12F catheter, thus improving the maneuverability of the snare within the vascular system. Curry30 later reported on a smaller but similar device constructed from a 0.021-inch or 0.028-inch guidewire and a 7 or 8F guiding catheter, respectively. All these devices used a snare oriented in line with the guiding catheter, which made retrieval of the foreign body somewhat difficult. FIGURE 51–1. (a) Proximal and (b) distal end of a J guidewire (arrows) extending across the right atrium from the inferior vena cava to the superior vena cava. This guidewire was ”lost” during placement of a femoral Shiley cathether. (c) Successful retrieval using a loop-snare device (arrow). In 1972, Randall24 proposed mounting a 0.018-inch loop at a 90-degree angle to the guidewire, thus facilitating greater coverage of the cross-sectional area of the vessel. Similar devices have since been described using guidewire loops constructed of 0.014-to 0.018-inch guidewires crimped or tied together and teamed with 8 to 14F guiding catheters, respectively.31,32 In 1992, Furui et al33 reported a loop-snare retrieval device constructed of a 7F, 80- or 110-cm-long, triplelumen catheter with a multipurpose configuration at the distal end. The largest of the three lumens was most distal and allowed for passage of a 0.035-inch guidewire for advancement and steering of the device. The other two lumens opened just proximal to the catheter’s tip. To create the loop snare, the two ends of a 0.018- to 0.021-inch guidewire were inserted into each lumen and brought out through the hub end of the catheter. The loop was crimped so that advancement of one end of the guidewire at the hub resulted in the snare deploying at a 90-degree angle to the guiding catheter. The most significant problem with all these devices is that, despite the fact that many are constructed of small-caliber guidewires, the loop remains relatively stiff and potentially traumatic, particularly at the point at which the guidewire has been crimped. In addition, the ”doubled-over” guidewire not only results in a high degree of internal friction but also adds to the bulkiness of the device, thus necessitating the use of a larger guiding catheter. In 1991, Yedlicka and co-workers reported a new right-angled snare (Amplatz Gooseneck Snare, Microvena, Vadnais Heights, MN) constructed of a braided nitinol cable (Fig. 51–2).34 Visualization of the loop is enhanced by gold-plated tungtsen coils, which are part of the nitinol cable. The snare does not use the thermal memory properties imparted to the nitinol inferior vena cava filter (Nitinol Medical Technologies, Woburn, MA); thus, no cooling or warming of the device is required. A monofilament nitinol wire is bonded to the cable shaft to increase the tensile strength. A thin-walled teflon shrink coat is used to bind the two wires and also helps to reduce internal friction when the cable shaft is moved back and forth within the guiding catheter. The nitinol cable has a tensile strength of 18 to 20 pounds compared with the 2 to 3 pounds of tensile rupture strength of stainless steel. The loop comes in 5,10, 15, 25, and 35 mm sizes. The first two designs use a 4F guiding catheter, and the three largest loops use a 6F guiding catheter. All five devices have a 100-cm working length. This new snare offers multiple advantages over the previously described snares, including (1) multiple preformed sizes (diameter loops) for maximum cross-sectional coverage of any size vessel; (2) lack of a sharp, potentially traumatic bend or crimp in the loop; (3) ultrahigh visibility; (4) maximum flexibility of both the loop and the braided guidewire; and (5) a preformed right-angle design without a significant transition zone between the loop and guiding cable. The only significant disadvantage of the gooseneck snare or any snare-type device is a necessity to be able to access a free end of the foreign body to initiate retrieval, which usually can be accomplished. In some situations, however, an alternative retrieval technique may need to be used first to reposition the foreign body so that a free end is accessible to the snare. In the initial article by Yedlicka et al,34 11 (85%) of 13 guidewire (0.018 to 0.035 inches) and catheter (5 to 8F) fragments were successfully retrieved from the central venous circulation (including the right heart and pulmonary arteries) in less than 15 minutes without complications. Since this article, numerous other investigators have reported excellent results with the gooseneck snare for foreign body retrieval in the vascular system.2,16–19 Cekirge et al2 reported a 100% clinical success rate in retrieving 13 intravascular foreign bodies.2 No procedural or postprocedural complications occurred, and all cases were completed within 30 to 40 minutes. FIGURE 51–2. The Arnplatz gooseneck nitinol snare; snare loop (arrowhead), guiding cable (short arrow), and guiding catheter (long arrow). A comparison of some of the available loop-snare devices is shown in Table 51–1. Intravascular foreign body retrieval using a loop-snare device is performed as follows: a femoral sheath is required in all cases to minimize trauma at the insertion site during catheter manipulations. In choosing a sheath, one must account for the fact that most catheter fragments, unless snared close to the free end, will be doubled over when removed. Thus, the sheath should be at least 4F larger than the fragment to be removed. The guiding catheter is advanced over a guidewire and positioned adjacent to the free end of the foreign body (Figs. 51–3 and 51–4). The guidewire is exchanged for the loop snare, which is passed through the guiding catheter and advanced out the distal end. After the loop snare opens within the vessel, the guiding cable is used to manipulate the snare in conjunction with the guiding catheter until the loop is positioned around the foreign body. The cable wire then is slowly withdrawn to close the loop around the foreign body, or the guiding catheter can be advanced gently over the cable wire to close the loop. With constant tension on the cable wire to hold the foreign body securely, the guiding catheter is withdrawn slowly and pulled through the sheath. One of the key advantages of the loop-snare technique is its ability to retrieve relatively large objects and significantly decrease in size the leading edge of the foreign body as the loop is drawn into the guiding catheter. This step allows for removal of large foreign bodies through a sheath markedly smaller than the foreign bodies’ ”open” diameter. Because many devices used in interventional radiology expand after deployment from the delivery system (i.e., embolization coils, vascular stents, inferior vena cava filters), the ability of a loop snare to reduce the diameter of the foreign body is paramount to achieving a less traumatic retrieval. Over the last 20 years, a significant number of case reports and small series have appeared in the literature documenting the successful retrieval of malpositioned, displaced, and migrated inferior vena cava filters using the loop-snare technique.13–16,35,36 In simplest terms, the loop snare is positioned over the filter and closed, pulling the expanded legs toward the central axis of the filter, thus allowing for safe repositioning or removal of the device through a sheath equal to or only slightly larger than that used for the initial delivery (Fig. 51–5). With the rapidly increasing use of intravascular stents, the same placement complications commonly seen with the17–19 inferior vena cava filters already have been documerited numerous times. In each case, the loop-snare method was used alone or in conjunction with a second device (i.e., tip deflecting wire, angioplasty balloon) to capture, reduce in diameter the leading edge of the stent, and either successfully reposition or remove the stent atraumatically (Fig. 51–6).

 Devices and Techniques for Foreign Body Removal Techniques
Devices and Techniques for Foreign Body Removal Techniques
Radiology Key
Fastest Radiology Insight Engine