12 Functional and Structural Abnormalities
Congenital Functional and Structural Abnormalities of the Spine
Spinal Arteriovenous Malformation
Frequency: rare; approximately 3–11% of intradural extramedullary masses.
Suggestive morphologic findings: an arteriovenous malformation (AVM) appears as a scalloped mass of very high density after bolus contrast administration. Early postinjection scans show rapid clearance of the contrast medium.
Procedure: contiguous thin slices before and after bolus contrast administration.
Other studies: magnetic resonance imaging (MRI) and spinal angiography. Myelography was the gold standard prior to MRI and spinal angiography, but today these studies are indispensable for diagnosis and treatment planning.
Checklist for scan interpretation:
 Extent of AVM?
Extent of AVM?
 Significant cord compression?
Significant cord compression?
 Evidence of prior hemorrhage?
Evidence of prior hemorrhage?
 Pathogenesis
Pathogenesis
The pathogenesis of arteriovenous malformations is controversial, but they are believed to result from anomalous vascular differentiation during about the sixth week of embryologic development. This leads to a persistence of thin-walled vessels with a deficient intima and media, primitive capillaries and precapillary channels, and arteriovenous shunts (Fig. 12.1).
Spinal AVMs are most commonly found on the dorsal aspect of the lower spinal cord. Embryologically, this has to do with the earlier differentiation of the ventral and rostral portions of the spinal vascular system. Spinal AVMs usually span a length of four or five vertebral segments, and may have one or more feeding arteries. They are classified into four types and numerous subtypes according to their location, extent, supply, and size. An AVM cannot usually be classified on the basis of the computed tomography (CT) findings, however.
The symptoms of spinal AVMs depend on their location and the associated change in intraspinal hemodynamics. Ischemic changes in the spinal cord may result from direct compression of the cord by the mass itself. They can also result from venous hypertension, with damming back of blood and outflow obstruction, or from an arterial steal effect. Hemorrhage, thrombosis, and water-hammer pulse have also been discussed as pathophysiologic factors. This explains why the location of the AVM does not always correlate with the location of the associated cord injury.
 Frequency
Frequency
Although they are rare, spinal AVMs are the most common spinal vascular anomaly. According to a study published in 1978, spinal AVMs make up approximately 3.3–11% of intradural extramedullary masses. Males predominate by a ratio of 4:1, and the peak occurrence is in the fourth and fifth decades.
 Clinical Manifestations
Clinical Manifestations
The initial symptoms typically appear between 35 and 40 years of age. In most cases they are indistinguishable from cord compression symptoms caused by other mass lesions. Pain is the most common initial symptom, and it often projects in a radicular pattern. Frequently the pain is worse at night and may increase after a hot bath. Approximately 65% of patients experience a symptom complex of sensorimotor disturbances in the lower extremities and anal sphincter dysfunction.
 CT Morphology
CT Morphology
A spinal AVM can be difficult to identify on unenhanced CT scans, on which it appears as an elongated, isodense intradural extramedullary mass. Following intravenous contrast administration, the AVM appears as a scalloped mass of very high density, often containing tortuous blood vessels that may extend over several segments. Whenever an AVM is suspected, the contrast medium should be administered by bolus injection, and the arterial phase of enhancement should be evaluated. AVMs are characterized by intense enhancement during the arterial phase and by a rapid fading of enhancement on early postinjection scans.
AVM appears on postmyelographic CT scans as a filling defect with the features described above.
Frequency: relatively common; often coexists with acquired abnormalities.
Suggestive morphologic findings: narrow spinal canal, short pedicles (anteroposterior diameter < 12 mm at cervical level, < 11.5 mm at lumbar level; low interpedicular distance 20 mm), decreased epidural fat.
Procedure: contiguous CT slices with secondary reconstructions.
Other studies: myelography has several applications in spinal stenosis, such as determining whether cerebrospinal fluid (CSF) exchange is maintained past the site of the stenosis. Myelography should be combined if possible with postmyelogram CT. With cervical stenosis, MRI is useful for detecting cervical myelopathy.
Checklist for scan interpretation:
 Degree and craniocaudal extent of the stenosis?
Degree and craniocaudal extent of the stenosis?
 Other circumscribed lesions (disk protrusions, spondylophytes) that could account for clinical symptoms?
Other circumscribed lesions (disk protrusions, spondylophytes) that could account for clinical symptoms?
 Facet hypertrophy?
Facet hypertrophy?
 Pathogenesis
Pathogenesis
Despite marked individual variations in the width of the cervical, thoracic, and lumbar spinal canal, numerous studies have been published on normal values, especially in the lumbar spine. The following minimum values are a useful guide for excluding spinal stenosis, regardless of the affected segment:
• The anteroposterior diameter should be at least 11.5 mm.
• The interpedicular distance should measure at least 16 mm.
• The width of the lateral recess should be no less than 3 mm.
• The area of the spinal canal should measure at least 1.45 cm2 in axial cross-section.
• The ligamenta flava should not exceed 4–5 mm in thickness.
For simplicity, it may be said that spinal stenosis is present whenever the spinal canal and lateral recess are disproportionately narrow in relation to the structures they contain.
Congenital spinal stenosis usually results from shortening of the pedicles. This may be an idiopathic condition or may result from a genetic disease such as achondroplasia. Symptoms are caused by a disproportion between the diameter of the dural sac and the width of the spinal canal or lateral recess. With achondroplasia, this disproportion typically increases in the craniocaudal direction, whereas in dysraphic syndromes and other conditions it may be circumscribed and confined to a few segments. Overall, however, congenital spinal stenosis is rare compared with acquired stenosis caused by degenerative diseases such as spondylarthrosis, facet hypertrophy, and disk protrusions. Many of these patients have a relative congenital narrowing that becomes symptomatic when a space-occupying condition supervenes. The symptoms may result from cord or nerve root compression, or from the compression of intraspinal blood vessels.
 Frequency
Frequency
Congenital spinal stenosis rarely occurs in isolation. Symptoms usually appear when acquired degenerative changes cause additional stenosis of a congenitally narrow spinal canal.
 Clinical Manifestations
Clinical Manifestations
Back pain is a common early symptom. Lumbar stenosis is frequently marked by spinal claudication, with a slowly progressive decline in walking distance. Bladder dysfunction and anal sphincter dysfunction may subsequently develop. Cervical stenosis may be associated with spinal ataxia and other signs of cervical myelopathy.
The classification of spinal stenosis is dis cussed in connection with acquired structural abnormalities on p. 357 (Fig. 12.2).
 CT Morphology
CT Morphology
Scans parallel to the disk spaces demonstrate narrowing of the spinal canal. The anteroposterior canal diameter should be at least 11.5 mm in the lumbar region and 12 mm in the cervical region. The interlaminar distance should be at least 16 mm, and the cross-sectional area at least 1.45 cm2. In addition, CT often shows a reduction or obliteration of epidural fat.
Spondylosis, Spondylolysis, Spondylolisthesis (Figs. 12.2–12.7)
Frequency: common.
Suggestive morphologic findings: osteophytic spurs and facet hypertrophy, leading to stenosis of the lateral recess and neuroforamina; a cleft defect in the pedicles (spondylolysis) or a step-off due to spondylolisthesis.
Procedure: CT may consist of contiguous slices or scans parallel to the disk spaces. Suspected spondylolysis is evaluated with reformatted images or direct scans parallel to the pedicles.
Other studies: MRI and radionuclide scans are useful for detecting spondylolysis and checking for activity or sclerosis of the bone margins. Compression of the subarachnoid space is evaluated by myelography and postmyelographic CT (Fig. 12.3a). Conventional radiographs. Function views.
Checklist for scan interpretation:
 Degree of stenosis?
Degree of stenosis?
 Degree of spondylolisthesis?
Degree of spondylolisthesis?
 Spondylolysis: is there a visible defect in the pedicles? Do adjacent portions of the pedicles have sclerotic margins?
Spondylolysis: is there a visible defect in the pedicles? Do adjacent portions of the pedicles have sclerotic margins?
 Pathogenesis
Pathogenesis
Spondylosis. This term is used for nonspecific degenerative spinal disease.
Spondylolisthesis. Spondylolisthesis refers to the anterior subluxation of one vertebral body over another. The most common form is displacement of L5 over S1, followed by L4 over L5.
Spondylolisthesis may result from spondylolysis, or a defect in the pedicles. Pseudospondylolisthesis can also occur due to degenerative or inflammatory changes in the facet joints (e.g., in patients with rheumatoid arthritis).
In the commonly used Meyerding classification, spondylolisthesis is divided into four grades, depending on the degree of vertebral body displacement, with each grade representing one-quarter of the longitudinal diameter of the displaced vertebral body (0–25% = grade I, > 75% = grade IV). This classification does not correlate with clinical manifestations, however (Fig. 12.3).
Complete slippage of one vertebral body over another is called spondyloptosis.
Spondylolysis. This refers to a unilateral or bilateral pars defect—i.e., a discontinuity in the part of the vertebral arch located between the superior and inferior articular processes. The defect may be congenital, but acquired forms can result from a posttraumatic nonunion or fatigue fracture of the vertebral arch (Fig. 12.5).
Most cases are asymptomatic or cause nonspecific symptoms in the form of recurring back pain. Rarely, spondylolysis can cause nerve root compression that requires surgical stabilization of the affected site.
Pars defects most commonly occur in the L5 vertebra, followed by L4. Spondylolysis is rare in the cervical vertebrae, where C6 is the most frequent site of occurrence. The linear defect or fracture line corresponds to the “collar” of the Lachapel dog figure in conventional oblique spinal radiographs.
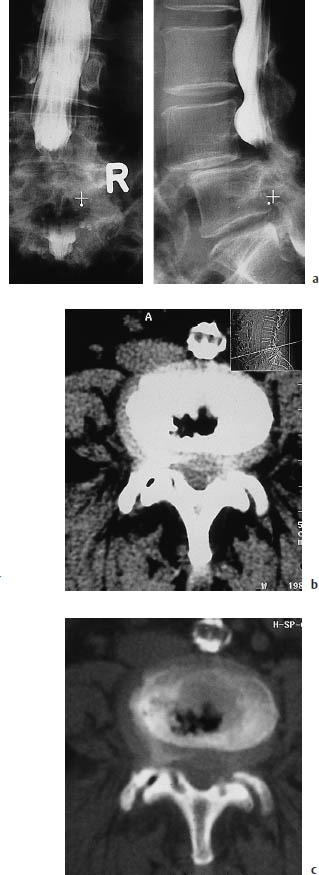
Fig. 12.3a–c Spondylolisthesis.
a Anteroposterior and lateral lumbar myelograms of a woman with grade II spondylolisthesis at L4–5 caused by bilateral spondylolysis of the L4 pedicles. The dural sac is severely compressed at the level of the step-off. Contrast medium injected above the stenosis can pass through the compressed site, but passage of contrast is considerably delayed, and it does not reach the caudal portions of the dural sac until the end of the examination.
b Axial CT scan through the affected level shows an apparently broad-based disk protrusion or excessive prominence of a real protrusion caused by the vertebral slippage. The lateral scout view, taken in the recumbent position, shows less slippage than the standing myelogram.
c The bone-window scan clearly demonstrates the excessive load placed on the intervertebral disk and facet joints by the hypermobility at the affected level. Note the clusters of nitrogen bubbles in the disk space and right facet joint.
Retrolisthesis. Retrolisthesis is the opposite of spondylolisthesis, denoting the posterior displacement of one vertebral body over another. Generally it does not result from a vertebral arch defect but from a decrease in the height of an intervertebral disk space. As the facet joints assume an oblique anterosuperior-to-posteroinferior orientation, the height reduction can lead to varying degrees of posterior slippage (Fig. 12.4).
 Frequency
Frequency
Nonspecific spondylosis is a very common degenerative condition. CT scans or radiographs demonstrate the changes in:
• 5–10% of patients 20–30 years of age
• > 50% of patients by age 45
• > 90% of patients by age 60
Spondylolisthesis, usually of low grade, is found in up to 20% of conventional lumbar spinal radiographs, according to Frymoyer (1988).
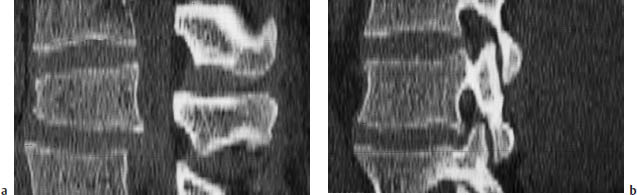
Fig. 12.4a, b Retrolisthesis. An L4–5 disk herniation has caused a substantial height reduction at the affected level.
a The pronounced retrolisthesis in the sagittal reformatted image is a result of the height reduction.
b The inferior articular process of L4 slips posteriorly downward on the superior articular process of L5, as on an inclined plane.
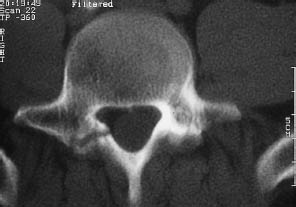
Fig. 12.5 Spondylolysis. Spondylolysis may be unilateral or bilateral and can result from trauma. Chronic overload can lead to a fatigue fracture, or a single traumatic event can fracture the vertebral arch, followed by the development of a nonunion. This image is a bone-window view of a right-sided spondylolysis caused by trauma.
Spondylolysis with a demonstrable pars defect is present of 4–7% of the general population and is frequently asymptomatic.
 Clinical Manifestations
Clinical Manifestations
Symptoms are usually nonspecific, and include the following:
• Low back pain
• Root compression syndromes
• Compression of the conus medullaris and cauda equina
Soft-tissue and bone-window CT scans will usually demonstrate the degenerative changes of spondylosis in the form of marginal bone spurs and hypertrophic facet joints. The radiologist needs to assess the extent to which these changes could produce clinically relevant central canal stenosis or lateral recess stenosis (see p. 337).
Spondylolisthesis is often apparent on CT scout views. It appears on single slices as an abrupt discontinuity between adjacent vertebral bodies. This can create the appearance of a broad-based protrusion of the intervening disk. (Caution: the degree of slippage may appear much greater in the standing patient and may become clinically relevant only in that position. We recommend comparing the scans with conventional standing radiographs or function views of the spine.)
Spondylolysis is best demonstrated by bone-window CT scans parallel to the pedicles, which slope slightly downward in an anterosuperior-to-posteroinferior direction. The gantry angulation should be adjusted accordingly (Fig. 12.6).
When the scans are interpreted, it may be important for the referring physician to know whether the margins of the pars defect are sclerotic. If they are not, there is a chance that nonoperative immobilization will be beneficial. It may be helpful to add a radionuclide bone scan when addressing this question. Intense tracer uptake, signifying active bone metabolism, again suggests that fusion of the site may be accomplished without operative treatment.
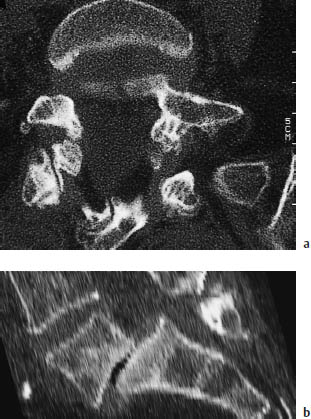
Fig. 12.6a, b Spondylolisthesis. Bilateral arch defects in L5 have led to a Meyerding grade II–III spondylolisthesis at the L5–S1 level.
a Axial bone-window scan.
b Sagittal reformatting.
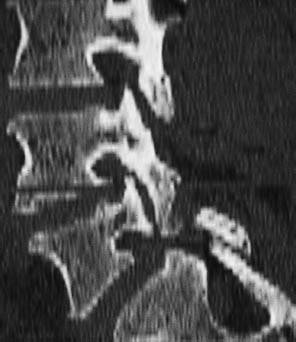
Fig. 12.7 Vertebral arch defects are often clearly visualized on sagittal CT reformatted images.
Syringomyelia, Hydromyelia (Figs. 12.8–12.10)
Frequency: often associated with other anomalies such as Arnold-Chiari malformation.
Suggestive morphologic findings: intramedullary area of low attenuation extending over several segments. The spinal cord may be expanded or atrophic. Postmyelographic CT may show rapid or delayed opacification of the canal by contrast medium.
Procedure: contiguous slices with secondary reconstructions. Postmyelographic CT can establish whether the syrinx communicates with the subarachnoid space.
Other studies: MRI (Figs. 12.9, 12.10), with a CSF pulsation study. Postmyelographic CT can determine the degree of CSF exchange with the subarachnoid space.
Checklist for scan interpretation:
 Longitudinal and lateral extent of the lesion?
Longitudinal and lateral extent of the lesion?
 Does the canal enhance on postmyelographic CT?When does this occur?
Does the canal enhance on postmyelographic CT?When does this occur?
 If CT findings are equivocal, proceed with MRI.
If CT findings are equivocal, proceed with MRI.
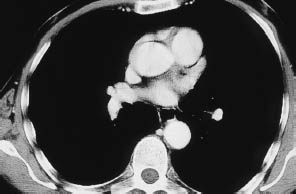
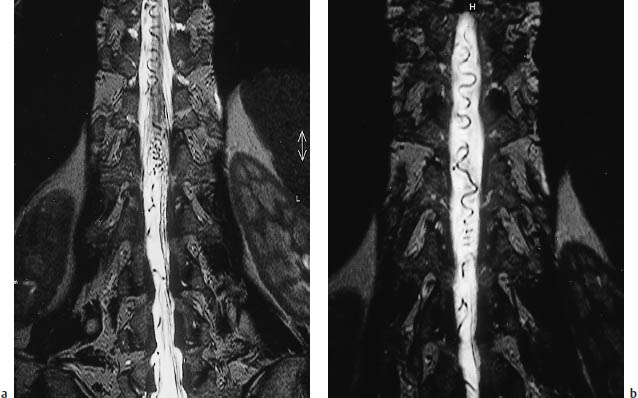
Fig. 12.8 Central hypodensity of the spinal cord. Thoracic CT scan in a man who sustained an L1 fracture about 10 years previously in a motor vehicle accident. For years, he had progressive lower extremity pain and weakness and bladder dysfunction. Even this survey scan, acquired for other reasons, clearly demonstrates a central hypodensity of the spinal cord. (The lesion was investigated further by magnetic resonance imaging.)
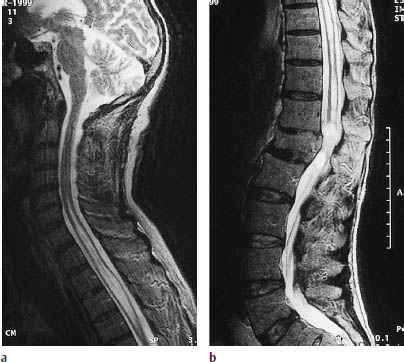
Fig. 12.9a, b Sagittal T2-weighted magnetic resonance imaging of the cervical spine and thoracolumbar junction area. The central canal of the spinal cord is expanded from the cervical cord to the level of L1. The height of L1 is reduced as the result of a fracture, which has caused impairment of cerebrospinal fluid circulation.
 Pathogenesis
Pathogenesis
Syringomyelia and hydromyelia are benign malformations of the spinal cord marked by the presence of an elongated, fluid-filled intramedullary cavity. Syringomyelia is distinguished by an intramedullary cavity that may be independent of the central canal, or may communicate with it but lacks an ependymal lining. Hydromyelia denotes persistence and expansion of the ependyma-lined central canal, which communicates with the fourth ventricle. The two anomalies are difficult to distinguish by CT in any given case. The central or lateral position of the expansion provides an uncertain clue to the identity of the lesion.
Both conditions can occur as a congenital or acquired anomaly of the spinal cord. Congenital forms usually occur in the setting of a complex syndrome. For example, from 20% to 70% of patients with Arnold-Chiari malformation have syringomyelia.
Other diseases that are frequently associated with syringomyelia or hydromyelia are as follows:
• Dysraphic disorders
• Dandy-Walker malformation
• Very severe scoliosis
• Klippel-Feil syndrome
Because the malformation is caused by altered hydrodynamics, it can develop in all diseases that lead to a congenital or acquired obstruction of CSF drainage.
The currently favored theory on the pathogenesis of syringomyelia is the hydrodissection theory, advanced by Williams et al. (1981). When actions occur that raise the intraabdominal pressure, such as coughing or sneezing, and when communication between the spinal and intracranial extra-axial CSF spaces is impaired (e.g., due to herniation of the cerebellar tonsils into the foramen magnum), a check-valve mechanism is created.
The intra-abdominal pressure is transmitted through epidural veins to the intraspinal space. This leads to an upward movement of the pressure wave, allowing CSF to flow into the cranial cavity. As the intraspinal pressure subsides, the check-valve mechanism prevents a compensatory downward pulsation in the extra-axial CSF spaces. The resulting negative pressure tends to increase the CSF pulsation in the central canal of the spinal cord (hydromyelia) and also increases interstitial fluid outflow, leading to the development of syringomyelia. Histopathologic studies of the cord in such cases have found dilated perivascular spaces. In many cases, the wall of the syrinx was rich in small arteries and veins.
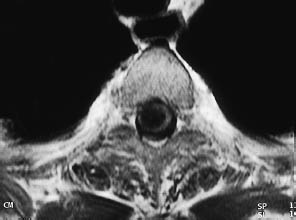
Fig. 12.10 Syringomyelia documented by axial T1-weighted magnetic resonance imaging.
This led Ball and Dayan (1972) to theorize that CSF and extracellular fluid enter the cavity through the Virchow-Robin spaces of the spinal cord. Another theory, advanced by Pillay et al. in 1992 and based largely on MRI studies, assumes that approximately 30% of CSF formation is intraspinal. Thus, any type of outflow obstruction caused by trauma or arachnoiditis, for example, can lead to intramedullary cavity formation by the mechanism described above.
 Frequency
Frequency
Syringomyelia or hydromyelia is commonly found in association with other anomalies.
 Clinical Manifestations
Clinical Manifestations
Many patients experience a gradual progression of the following neurologic symptoms:
• Dissociated sensory loss at the level of the affected segments
• Motor disturbances and weakness mainly affecting the upper extremity
• Very severe pain in some cases
CT sometimes demonstrates a circumscribed, elongated region of low attenuation within the spinal cord (Fig. 12.8). The cord may be expanded or atrophic. The cavity may already occupy most of the cord diameter. There is frequent widening of the bony spinal canal. On postmyelographic CT, the canal may enhance rapidly through direct connections with the subarachnoid space, or it may opacify slowly over 4–8 hours due to gradual contrast permeation.
Klippel-Feil Syndrome (Figs. 12.11, 12.12)
Frequency: rare.
Suggestive morphologic findings: the fusion of two or more vertebral bodies (Fig. 12.11).
Procedure: contiguous thin CT slices with secondary reconstructions.
Other studies: conventional radiographs. MRI can evaluate for cervical myelopathy.
Checklist for scan interpretation:
 Craniocaudal extent of the fusion?
Craniocaudal extent of the fusion?
 Evaluate the remaining unaffected segments, since compensatory hypermobility leads to early degenerative changes that may cause clinical symptoms.
Evaluate the remaining unaffected segments, since compensatory hypermobility leads to early degenerative changes that may cause clinical symptoms.
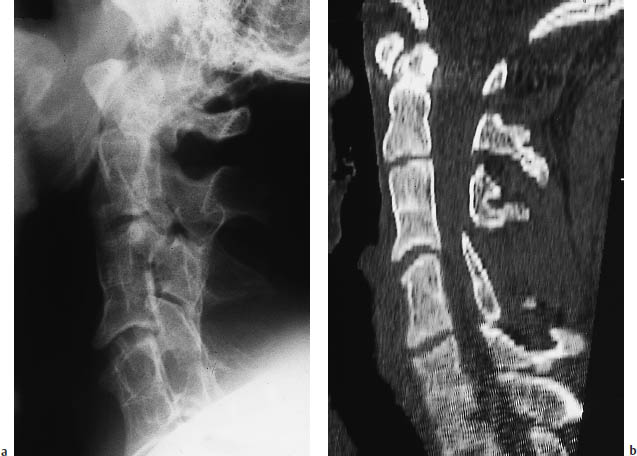
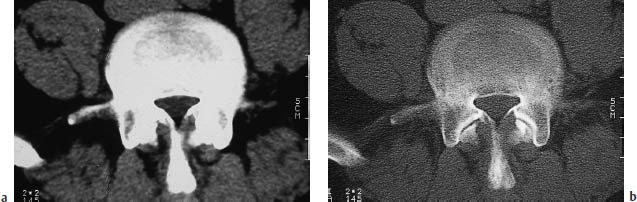
Fig. 12.11a, b Klippel-Feil syndrome as demonstrated by a conventional radiograph and sagittal CT reformatted image of the cervical spine. The images show interbody fusion and atypical segmentation of the entire cervical spine, manifested clinically by a profound limitation of neck motion in this young patient.
Klippel-Feil syndrome is based on the congenital fusion of two or more cervical vertebrae. The fusion may be limited to the vertebral bodies or may affect the entire vertebra including the posterior elements. In most cases the vertebrae are flattened, and the associated disk spaces are absent or hypoplastic. The neuroforamina are narrowed. Patients with Klippel-Feil syndrome often have coexisting clinical abnormalities.
The classic triad of the Klippel-Feil syndrome consists of:
• Short neck
• Limited motion of the cervical spine
• Low nuchal hairline
Less than 50% of patients exhibit all three of these features, however.
Other associated anomalies are listed below:
• Platybasia
• Basilar impression
• Syringomyelia
• Encephalocele
• Cranial asymmetries
• Syndactyly
• Renal anomalies
• Anal atresia
• Cardiac anomalies
• Supernumerary pulmonary lobes
• Cleft lip and palate
• Deafness due to inner ear anomalies
Another frequent anomaly, found in approximately 25–40% of cases, is a unilateral elevation of the scapula (Sprengel’s deformity).
Klippel-Feil syndrome is probably based on a hereditary developmental error, with deficient segmentation of the cervical somites between the third and eighth weeks of embryonic development. Similar changes, including interbody fusion, are also found in children with alcoholic embryopathy or chromosome abnormalities (Figs. 12.11, 12.12).
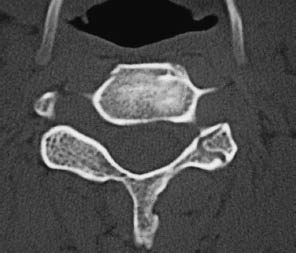
Fig. 12.12 Block vertebrae in Klippel-Feil syndrome. Besides absence of the disk space, single axial CT slices show partial assimilation of the facet joints, which is typical of the partial or complete fusion of adjacent vertebral bodies.
 Frequency
Frequency
Klippel-Feil syndrome is rare. Its true incidence is unknown, since many cases of block vertebrae are asymptomatic.
 Clinical Manifestations
Clinical Manifestations
Most cases are asymptomatic. When symptoms occur, they usually result from early osteoarthritic degeneration of the hypermobile adjacent segments. Rarely, compression syndromes of the cervical cord or nerve roots are observed.
 CT Morphology
CT Morphology
CT demonstrates the fusion of two or more cervical vertebrae.
Spina Bifida
Frequency: a common incidental finding.
Suggestive morphologic findings: incomplete closure of the vertebral arch with no associated meningeal anomaly.
Procedure: obtain secondary reconstructions only if the defect is clinically relevant.
Other studies: conventional radiographs. MRI may be helpful to exclude involvement of the meninges and spinal cord.
Checklist for scan interpretation:
 Extent of the defect?
Extent of the defect?
 Is the defect limited to bony structures?
Is the defect limited to bony structures?
 Check for associated anomalies (lipomas, etc.).
Check for associated anomalies (lipomas, etc.).
 Pathogenesis
Pathogenesis
Spina bifida is based on a congenital absence of the spinous processes and portions of the vertebral arch. It is not associated with meningeal or cord protrusion. In some cases, the defect can be detected by external palpation.
 Frequency
Frequency
Spina bifida is a common defect. Mild forms are detected in approximately 20–30% of the population.
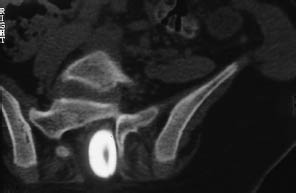
Fig. 12.13 Spina bifida. A postmyelographic CT in sacral spina bifida demonstrates a meningocele plus dorsal adhesion of the filum terminale.
 Clinical Manifestations
Clinical Manifestations
Spina bifida is not necessarily associated with neurologic deficits. Often the diagnosis is made incidentally or is suggested by coexisting anomalies such as diastematomyelia, lipoma, tethered cord syndrome, dermoid, or by a hairy nevus overlying the defect.
 CT Morphology
CT Morphology
CT shows incomplete closure of the neural arch with no accompanying anomaly of the meninges, underlying cord, or nerve roots (Fig. 12.13).
If spina bifida is detected incidentally, no further studies are necessary.
Myelomeningocele
Frequency: common.
Suggestive morphologic findings: herniation of leptomeninges and variable neural elements through a bony defect.
Procedure: contiguous CT slices with secondary reconstructions.
Other studies: MRI to demonstrate the entire neural axis in sagittal section (often permits better evaluation of associated anomalies).
Checklist for scan interpretation:
 Location and extent of the myelomeningocele?
Location and extent of the myelomeningocele?
 Any evidence that the lesion has ruptured?
Any evidence that the lesion has ruptured?
 Pathogenesis
Pathogenesis
Myelomeningocele is a dysraphic anomaly in which a sac covered by leptomeninges and containing CSF and variable neural elements herniates through a bony defect in the spine. If the sac does not contain neural elements, it is classified as a meningocele.
As in spina bifida, the etiology of myelomeningocele is based on a neural tube defect in the area of the caudal neuropore, which normally closes by the 28th day of embryologic development. Myelomeningocele is frequently associated with other anomalies such as Arnold-Chiari malformation, congenital scoliosis, vertebral anomalies, syringomyelia or hydromyelia, and tethered cord syndrome.
A special form is traumatic meningocele following the avulsion of nerve roots (e.g., from the brachial plexus).
 Frequency
Frequency
With an incidence of one per 1000–2000 live births, myelomeningocele is the most common congenital anomaly of the central nervous system (CNS).
 Clinical Manifestations
Clinical Manifestations
The clinical symptoms depend on the size and location of the myelomeningocele. High cervical lesions can cause complete lower extremity paralysis in severe cases.
 CT Morphology
CT Morphology
CT demonstrates the herniation of leptomeninges and variable nerve structures through a bony defect in the spine. The defect is usually located in the dorsal lumbosacral or suboccipital area and is associated with widening of the spinal canal at that level. Special forms are anterior sacral myelomeningocele and lateral thoracic meningocele.
Tethered Cord Syndrome
Frequency: often accompanies myelomeningocele and other syndromes.
Suggestive morphologic findings: low conus medullaris, thickened filum terminale, visible fatty tissue at the site of attachment.
Procedure: postmyelographic CT is ideal for positively identifying the filum terminale and its site of attachment (Figs. 12.13, 12.14).
Other studies: sagittal MRI is useful for detecting signs of myelopathy.
Checklist for scan interpretation:
 Level of the conus medullaris in relation to the lumbar spine?
Level of the conus medullaris in relation to the lumbar spine?
 Diameter of the filum terminale?
Diameter of the filum terminale?
 Coexisting anomalies?
Coexisting anomalies?
 Pathogenesis
Pathogenesis
Tethered cord syndrome involves a low position of the conus medullaris combined with a short, thickened filum terminale. It occurs when the dural attachment of the filum terminale acts as a checkrein to the physiologic ascent of the cord. Often a lipoma is found at the broad-based site of attachment. As the vertebral column grows in length, it exerts traction on the spinal cord leading to chronic recurring ischemia.
Tethered cord syndrome is commonly associated with myelomeningocele.
 Frequency
Frequency
The majority of patients with myelomeningocele also have radiologic evidence of tethered cord syndrome.
 Clinical Manifestations
Clinical Manifestations
The most common symptoms are:
– Pain
– Accompanying anomalies such as scoliosis and foot deformities
Many patients also have motor deficits such as unsteady gait, micturition difficulties, and incontinence. Skin changes such as nevi and hairy patches are often found over the defect.
Tethered cord syndrome is assumed to be present in all myelomeningocele patients who develop scoliosis, gait deterioration, progressive lower extremity spasticity, or voiding difficulties and pain. These developing symptoms usually permit a diagnosis to be made between 5 and 15 years of age.
 CT Morphology
CT Morphology
Even without contrast administration, CT can demonstrate a lipoma at the site where the filum is tethered. A low conus medullaris combined with a thickened filum terminale (> 2 mm in diameter) confirms the diagnosis, but postmyelographic CT is the only imaging study that can unequivocally define the tethered filum (Fig. 12.14).
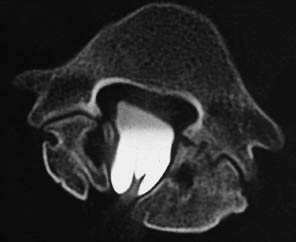
Correct window and center settings (e.g., W= 2500, C = 1000) should be used to reduce partial volume effects caused by the surrounding contrast medium.
Diastematomyelia (Myeloschisis) (Figs. 12.15–12.17)
Frequency: rare.
Suggestive morphologic findings: a bony or fibrous septum splitting the spinal cord into halves in the sagittal plane.
Procedure: contiguous thin CT slices, secondary reconstructions, bone window.
Other studies: MRI, postmyelographic CT. Unlike MRI, CT can distinguish between a bony and fibrous septum.
Checklist for scan interpretation:
 Height and extent of the lesion? Composition of the septum?
Height and extent of the lesion? Composition of the septum?
 Are there any other anomalies that could affect operative treatment (e.g., tethered cord syndrome)?
Are there any other anomalies that could affect operative treatment (e.g., tethered cord syndrome)?
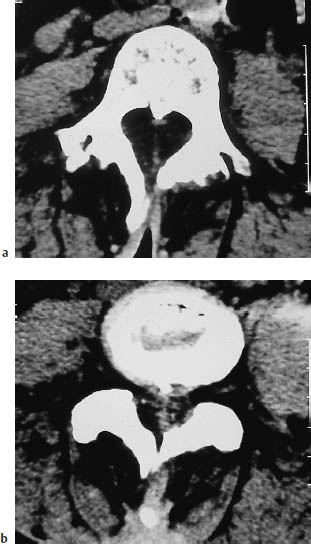
Fig. 12.15a, b Diastematomyelia. In diastematomyelia, the affected portion of the spinal cord is split into two separate parts by a midline septum. The septum may be fibrous, as in this example, or may consist of a bony spur (see Fig. 12.16).
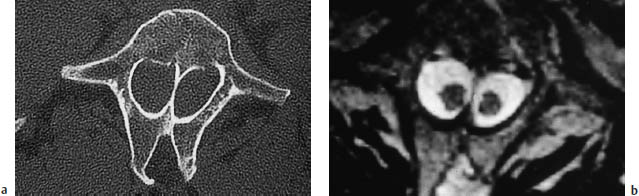
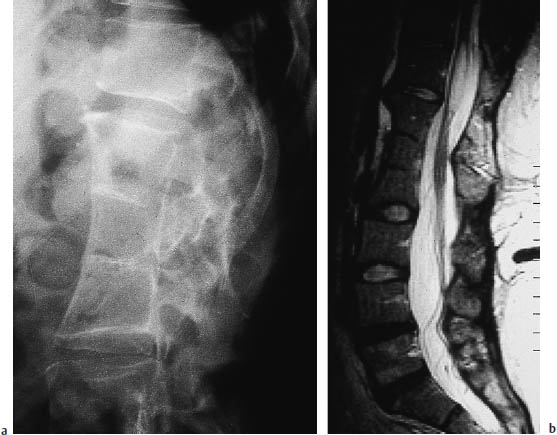
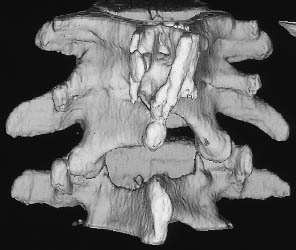
Fig. 12.17a–c Diastematomyelia (same patient as in Fig. 12.16). Fusion of the T12–L3 vertebral bodies is seen in a conventional radiograph (a), sagittal T2-weighted magnetic resonance image (b), and in a shaded surface display reconstructed from CT slices (c).
Diastematomyelia is a congenital anomaly that occurs during embryologic development when the neural tube becomes folded over due to adhesions between the ectoderm and endoderm. This leads to a division of the spinal cord, conus medullaris, and filum terminale by a fibrous membrane (25 %) or bony septum (75 %) located in the midsagittal plane. Diastematomyelia is frequently accompanied by myelomeningocele, tethered cord syndrome, and congenital scoliosis, and is associated with clinical stigmata such as hairy patches on the back and foot deformities. The condition most commonly occurs in the lower thoracic and upper lumbar regions. It is common to find a marked decrease in vertebral height or complete absence of the disk spaces at the level of the defect. Interbody vertebral fusion is also common. A variant of diastematomyelia is diplomyelia, in which two separate hemicords occupy a common dural sac with no demonstrable fibrous or bony septum.
 Frequency
Frequency
Diastematomyelia is a rare anomaly. Females are predominantly affected, by a ratio of about 3:1.
 Clinical Manifestations
Clinical Manifestations
The clinical symptoms are very similar to those that may occur in tethered cord syndrome.
 CT Morphology
CT Morphology
CT usually shows a conspicuous division of the spinal cord along the sagittal plane. The division is effected by a bony or fibrous septum and is almost always associated with other anomalies.
Lipomyeloschisis (Dural Lipoma, Lipomyelomeningocele, Fibrolipoma of the Filum Terminale)
Frequency: common in the setting of complex dysraphisms.
Suggestive morphologic findings: intramedullary, intradural or extradural mass of fat density, often in the setting of dysraphic pathology.
Procedure: contiguous CT slices, especially in complex malformations.
Other studies: MRI to define the extent of the anomaly in the sagittal plane.
Checklist for scan interpretation:
 Location in relation to a lumbar or sacral vertebral body?
Location in relation to a lumbar or sacral vertebral body?
 Extent and degree of the anomaly?
Extent and degree of the anomaly?
 Pathogenesis
Pathogenesis
The dysraphic disorders include the various forms of lipomyeloschisis:
• Intradural lipoma
• Lipomyelomeningocele
• Fibrolipoma of the filum terminale
These anomalies are typically associated with a low position of the conus medullaris, a posterior neural arch defect affecting one or more vertebral bodies, and changes in the overlying skin.
Most lipomas occur in the extradural space of the lumbosacral area, where the lesion often extends into the subcutaneous tissue. Lipomas can occur anywhere along the spine, however, and may occasionally affect the entire spinal canal. Intradural and even intramedullary occurrence has also been described.
 Frequency
Frequency
Lipomyeloschisis often occurs in the setting of a complex dysraphism. Intraspinal lipomas may also occur independently of other anomalies, and some do not become symptomatic until adulthood. Males and females are affected equally.
 Clinical Manifestations
Clinical Manifestations
If a complex malformation is present, its severity determines the clinical presentation. The dominant features usually consist of motor and sensory disturbances in the lower extremity and bladder dysfunction.
 CT Morphology
CT Morphology
CT scans show an intramedullary, intradural, or extramedullary mass of fat attenuation (–100 HU) that does not enhance after contrast administration. Frequently these masses are found in the setting of a complex dysraphic disorder. For example, they may occur at the level of attachment of the filum terminale in a patient with tethered cord syndrome (Fig. 12.18).
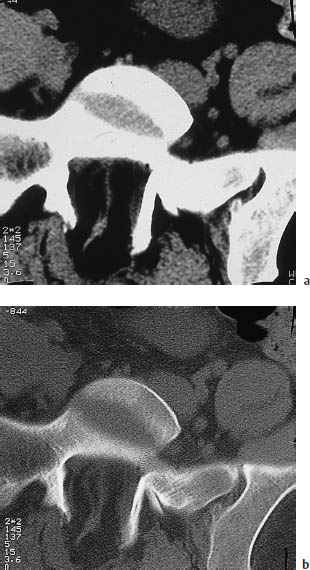
Fig. 12.18 Lipomyeloschisis. CT scans at the S1 level show a congenital neural arch defect accompanied by dorsal tethering of the filum terminale and an intraspinal lipoma.
a Soft-tissue window.
b Bone window.
Spinal Meningeal Cysts (Figs. 12.19, 12.20)
Frequency: common incidental finding.
Suggestive morphologic findings: variable-size cystic mass of CSF density, which may compress the dural sac or individual nerve roots and may erode bony structures.
Procedure: postmyelographic CT is best for determining whether the cyst communicates with the rest of the subarachnoid space.
Other studies: myelography (type I and II cysts appear as contrast-filled outpouchings). MRI shows a cystic mass whose contents are isointense to CSF (Fig. 12.20).
Checklist for scan interpretation:
 Spinal meningeal cysts are usually detected incidentally.
Spinal meningeal cysts are usually detected incidentally.
 Nerve root compression?
Nerve root compression?
 Does the cyst envelop the nerve root?
Does the cyst envelop the nerve root?
 Pathogenesis
Pathogenesis
Spinal meningeal cyst is the term applied to cystic or diverticular, intradural and extradural outpouchings of the spinal meninges. They are known by various other names as well:
• Tarlov cyst
• Spinal arachnoid cyst
• Dural diverticulum
• Root sheath cyst
A classification proposed in 1988 is shown in Table 12.1.
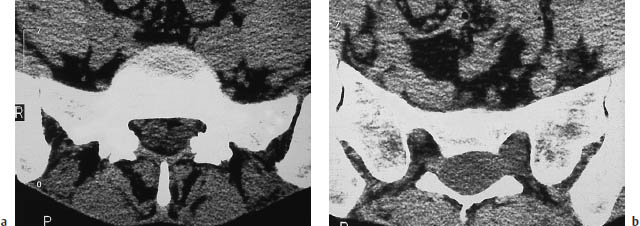
Fig. 12.19a, b Spinal meningeal cyst. CT demonstrates a sacral extradural mass displacing the dural sac and nerve roots and almost completely filling the sacral spinal canal. Note how the cyst had displaced the left S1 nerve root against the posterior surface of the neuroforamen. This classifies the lesion as a type Ia spinal meningeal cyst that does not contain a nerve root (see Table 12.1).
| Type | Description | |
|---|---|---|
| I | Extradural meningeal cyst that does not contain a nerve root | |
| Ia Extradural meningeal cyst | ||
| Ib Occult sacral meningocele | ||
| II | Extradural meningeal cyst that contains a nerve root (perineural Tarlov cyst, root-sheath cyst) | |
| III | Intradural arachnoid cyst |
 Frequency
Frequency
Spinal meningeal cysts are a common incidental finding, especially in postmyelographic CT.
 Clinical Manifestations
Clinical Manifestations
Most spinal meningeal cysts are asymptomatic. If large enough, however, type I cysts in particular can cause root compression with associated symptoms.
 CT Morphology
CT Morphology
CT demonstrates a cystic mass of variable size that arises from the meninges and is filled with fluid of CSF density. In postmyelographic CT scans of type I and II cysts, the contrast distribution always confirms a communication between the cyst and subarachnoid space. Type III cysts most commonly occur in the dorsal subarachnoid space. Larger lesions of long duration can cause bone erosion with widening of the spinal canal and neuroforamina (Fig. 12.19).
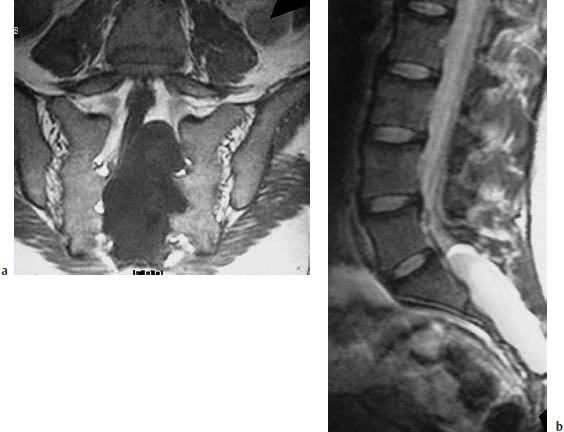
Fig. 12.20 Spinal meningeal cyst.
a The T1-weighted coronal magnetic resonance image (MRI) defines the full craniocaudal extent of the cyst, and reveals its mass effect on the dural sac and nerve roots.
b The T2-weighted sagittal MRI reconfirms the cystic nature of the mass. The cyst contents are isointense to cerebrospinal fluid in both the T1-weighted and T2-weighted images.
Acquired Functional and Structural Abnormalities of the Spine
Spinal Trauma
Fractures (Figs. 12.21–12.27)
Frequency: common.
Suggestive morphologic findings: step-off or contour irregularity; fragmentation, attenuation, irregularity, or fine linear disruption of the trabecular architecture. Associated soft-tissue injuries, paravertebral or intraspinal hematoma.
Procedure: contiguous thin CT slices, secondary reconstructions, bone window.
Other studies: compare CT findings with conventional radiographs.
Checklist for scan interpretation:
 Fracture type?
Fracture type?
 Signs of displacement or instability?
Signs of displacement or instability?
 Extent of associated soft-tissue injuries?
Extent of associated soft-tissue injuries?
 Narrowing of the spinal canal?
Narrowing of the spinal canal?
 Compression of the dural sac?
Compression of the dural sac?
 Describe the fracture.
Describe the fracture.
 Evaluate for possible instability using the three-column model.
Evaluate for possible instability using the three-column model.
There are various ways that CT can be used in the evaluation of spinal fractures. The choice in a given case depends mainly on the communication between the radiologist and the traumatologist at the facility where the patient has been admitted.
Frequently, conventional plain radiographs are the first step in the radiologic work-up of spinal trauma patients. Levels at which plain films show a fracture or possible structural injury are then evaluated with thin axial CT slices. If conventional radiographs are negative, CT scans are obtained at the levels that correlate with presenting neurologic deficits.
CT is the initial examination at some trauma centers, especially in multiple injury patients, in whom it can be used to screen for associated injuries to internal organs as well as bony injuries to the pelvis and spine. If the initial findings are abnormal or suspicious, the affected spinal segments can be selectively reevaluated using thin CT slices. This study may include scans acquired with parallel gantry angulation to the disk spaces.
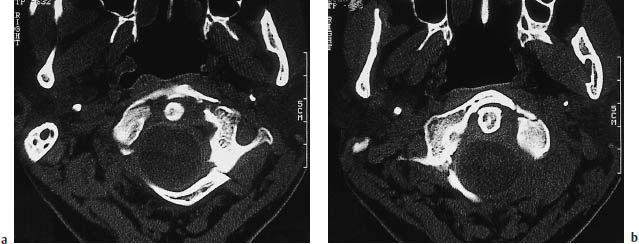
Fig. 12.21a, b Jefferson fracture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






