Introduction
Magnetic resonance imaging (MRI) is very adept at displaying structural anatomy of the brain in great detail. However, the correlation between anatomy and function is not always clear or apparent, and this becomes particularly difficult in situations where the anatomy deviates from the expected owing to developmental anatomic variation or if there is structural distortion by disease.
A few short years after the clinical use of MRI for imaging the human brain, Ogawa et al. described the imaging technique they called blood oxygenation level–dependent (BOLD) contrast for MRI. They demonstrated that the sensitivity to paramagnetic effects of deoxyhemoglobin could be increased with the use of gradient echo (GRE) techniques and that this BOLD contrast followed physiologic changes and could provide in vivo real-time maps of blood oxygenation. The initial studies described drug-induced changes, but this was soon followed by studies describing the BOLD response to physiologic visual and primary sensory activation by two groups almost simultaneously in 1992. This BOLD technique is the most commonly used method for functional MRI (fMRI).
Hemodynamic Response Function
BOLD fMRI measures or demonstrates the effects of neuronal activity via the changes in the hemodynamic correlate. Deoxyhemoglobin is paramagnetic, whereas oxyhemoglobin is diamagnetic. The presence of paramagnetic deoxyhemoglobin in a blood vessel results in local susceptibility differences that produce signal decreases (hypointensity) on GRE T2* images. Because oxyhemoglobin is diamagnetic and does not cause similar susceptibility changes, the changes in oxygenation of blood can be observed as signal changes on the T2* images.
On the onset of neural activity, because oxygen consumption is expected to increase, it is also expected that local deoxyhemoglobin concentration would increase and result in decreased MRI signal. However, following a small transient decrease in BOLD signal at the onset of neural activity, it has been observed that there is a much more pronounced increase in signal, implying a net decrease in deoxyhemoglobin concentration. This has been thought to result from a much larger increase in cerebral blood flow in response to the initial increased oxygen extraction, bringing in more oxygenated blood (oxyhemoglobin), with consequent reduction in deoxyhemoglobin levels in the local voxels. This variation of the BOLD fMRI signal as a function of time in response to neuronal activity is known as the hemodynamic response function (HRF). The relationship between the underlying neural activity and the resultant hemodynamic response is also known as neurovascular coupling.
BOLD signal changes occur in tissue (extravascular BOLD effect) as well as within the vasculature (intravascular), with additional signal changes also occurring within the larger vessels.
Although the physics behind the BOLD response is fairly well understood, there have been numerous theories proposed to explain the neural basis of the BOLD response. The favored explanation is a relatively direct correlation between fMRI signal and synaptic activity via a neurotransmitter-driven hyperemia response.
Clinical fMRI
Most fMRI studies performed for clinical use are task-based studies that require the patient to perform a motor, language, or visual task in the MRI scanner while (typically) GRE T2*-weighted echo planar images (EPI) are rapidly acquired. Another less clinically established but promising BOLD technique that will be discussed later in this chapter is resting state functional MRI (rs-fMRI).
Task-Based fMRI
Most fMRI performed clinically is task based, with ultrafast images (e.g., single-shot T2* GRE echo planar technique) obtained during performance of defined motor or sensorimotor, language, or other cognitive or visual tasks. The magnitude of the BOLD signal changes is relatively small and can be more pronounced on high-field-strength magnets (at our institution, we perform our fMRI studies on a 3-tesla [T] scanner). Use of a block design for the fMRI examination (described later) and multiple repetitions also improve signal-to-noise ratio and BOLD contrast.
The rapid EPI technique allows imaging of the whole brain with a temporal resolution of approximately 2000 milliseconds and a spatial resolution of approximately 3 to 5 mm. A typical fMRI study paradigm would acquire generally between 90 and 120 whole brain volumes in 3 to 4 minutes. Disadvantages of the EPI technique include heightened susceptibility artifacts, especially at the skull base. Spin echo methods with acquisition of T2-weighted images can also be employed to minimize these artifacts; however, they provide lower BOLD effect and lower contrast between images acquired during rest and activity states.
The stimulus or the activation paradigms are essentially of two types: block design paradigms or event-related paradigms. Block design paradigms consist of alternating blocks/epochs of a previously defined task alternating with equal-duration blocks/epochs of rest or a control task ( Fig. 16-1 ). In most sensorimotor or language paradigms, each epoch duration in a block design is approximately 20 to 30 seconds. Event-related paradigms or single event paradigms are designed with short periods of activation alternating with longer periods of rest, with MR acquisition methods designed to measure hemodynamic changes over several seconds. Advantages of event-related design include the ability to present very brief stimuli at irregular intervals. Disadvantages of such design include greater task difficulty, particularly for neurologically impaired patients, and much lower statistical power for equivalent-duration paradigms. In clinical fMRI, block design paradigms are generally used because they are generally easier to perform, are more efficient, and produce more statistically robust results.

Motor Tasks.
All the motor tasks we use are designed as a block design paradigm, with 30 seconds of rest alternating with 30 seconds of activity within each cycle, with the visual (or aural) instructions to “stop” or “go.” The most common sensorimotor tasks include a foot movement task (with continuous bilateral gentle plantar flexion and dorsiflexion of the foot at the ankle at a steady pace, or alternatively toe flexion and extension), a finger movement task (which includes bilateral simultaneous sequential finger tapping), and a vertical tongue-movement task (with continuous gentle up-and-down movement of the tongue at a steady pace, with special care to avoid movement of the jaw) that elicits activation related to the face motor area. Alternatively, lip-puckering may be used for eliciting activation in the lower face sensorimotor cortex. A variety of other hand movement tasks (e.g., hand opening/closing, squeezing) can be used to activate the hand representation area of the primary motor cortex.
If one thinks of the diagrammatic representations of the motor homunculus from physiology textbooks, the hand representation is always displayed to be overrepresented and takes up a large portion of the motor strip. Because the hand motor tasks are relatively simple to perform and show good activation of the motor cortex, hand motor paradigms should be performed if the primary motor cortex is to be localized. The bilateral finger-tapping task is successful in eliciting activation of the primary motor as well as the somatosensory cortex of the precentral and postcentral gyrus, respectively, in addition to the supplementary motor area (SMA) along the medial aspect of the cerebral hemisphere (see Fig. 16-3 ).
We often employ a more complex three-phase hand opening/closing task (right hand followed by left hand and then by a rest phase) using a dynamic visual display as the cue to obtain motor cortical activation maps in the precentral gyrus while minimizing sensory cortex activation ( Fig. 16-2 ).

The foot/ankle movement task results in robust activity within the precentral gyrus near the superior termination of the central sulcus and more prominently in the paracentral lobule, with activation of the supplementary motor area.
Cerebellar activation is commonly observed during motor tasks in the hemisphere contralateral to the activated primary motor cortex.
Language Tasks.
Functional MRI has been shown to be reliable for the prediction of hemispheric language dominance. Numerous paradigms are typically used to elicit the activation in eloquent cortex related to language function. Certain paradigms have been specially designed to elicit activation primarily in the speech-production areas (expressive paradigms) in the receptive language regions (receptive paradigms), whereas others activate both regions. Language-activation paradigms may be further divided into semantic, phonologic, or verbal fluency paradigms. In general, for clinical language fMRI, only covert (silently thought) rather than overt (physically spoken) language tasks are used to minimize head/mandibular motion. The expressive paradigms have been designed to elicit activation in the inferior frontal gyrus (IFG) corresponding to the region of Broca’s area, the dorsolateral prefrontal cortex (DLPFC) over the convexity of the frontal lobe, usually in the hemisphere dominant for language, and in the premotor or language supplementary motor area (pre-SMA) along the medial aspect of the frontal lobes ( Fig. 16-3 ).
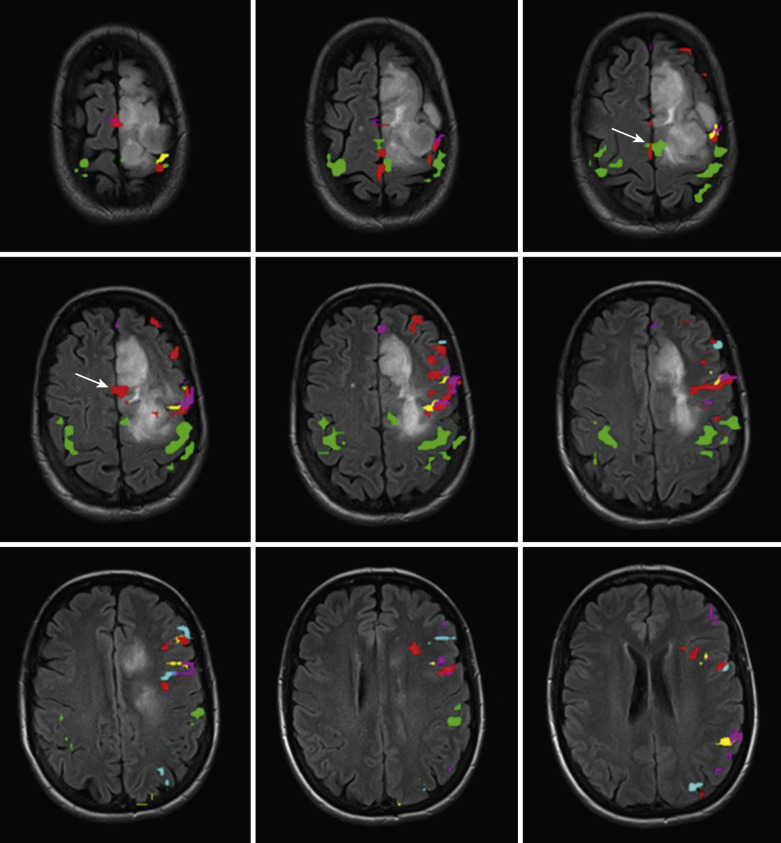
The receptive paradigms have been designed to elicit activation in the receptive language areas predominantly in the temporal and parietal lobes, most prominently in Wernicke’s area, which technically involves Brodmann area 22 in the posterior-most aspect of the superior temporal gyrus (STG), although activation is typically seen along the posterior aspect of the superior temporal sulcus (STS), middle temporal gyrus (MTG), and temporal occipital junction, as well as in the inferior parietal lobule, including the angular and supramarginal gyrus (SMG) ( Fig. 16-4 ).

Expressive paradigms.
Some of the expressive language tasks commonly used at our institution include covert word-generation tasks such as the silent word–generation task and the object-naming task. During the silent word–generation task, the patient is asked to covertly generate multiple words starting with the presented letter of the alphabet. In the object-naming task, the patient is asked to silently name a series of presented objects. The control task uses a nonsense drawing for visual fixation in order to minimize the effects of visual cortex activation.
Silent word generation has been compared favorably with the Wada test in the lateralization of language function. With this task, activation is typically identified within the DLPFC and IFG, and variably within the cingulate language regions, as well as in the pre-SMA regions. This task produces robust activation and effectively lateralizes speech functions. A similar task is a verb-generation task where instead of a letter of the alphabet, clues are visually or aurally presented. The patient is then asked to generate related verbs.
Object naming is a simple covert word-generation task intended to activate language cortical functions of the dominant hemisphere, including in the IFG in addition to other areas.
An example of a phonologic task is the rhyming task. The more complex rhyming task alternates epochs of a series of word pairs with a series of images showing two rows of stick figures, where the subjects respond electronically if the word pairs rhyme or if the rows of stick figures match. Typical activation includes DLPFC, IFG, STG, and STS.
Receptive paradigms.
Examples of commonly used receptive (i.e., comprehension or semantic) paradigms include the sentence listening comprehension and sentence reading comprehension tasks, as well as the passive story-listening task.
The sentence listening comprehension task alternates blocks of real sentences with garbled speech and requires the subject to respond via button presses on a keypad only if the content of the presented sentence is true. The reading comprehension task is a similar paradigm that uses written sentences instead of aural stimuli. These are useful for clinical questions regarding language localization in Broca’s area and Wernicke’s area.
Another example of a simple receptive task is a passive listening task that alternates segments of a short story with garbled speech sounds. Activation is most typically seen within the STG and in the cortex lining the STS. Additionally, activation is seen within the STG and MTG, more anteriorly.
Semantic paradigms.
Semantic paradigms are more general purpose for language mapping than the expressive and receptive paradigms described. These are designed to be useful for language lateralization as well as localization of key expressive and receptive speech areas in the dominant hemisphere.
The sentence completion task requires the patient to covertly complete written sentences that have been displayed with the last word missing. The control block consists of simple designs or a nonsense series of scrambled letters arranged in the form of a sentence. For this task, the dominant IFG activation is seen, consistent with the dominant expressive speech area, together with left MTG activation, consistent with the dominant receptive speech area.
There are other semantic decision paradigms such as noun-verb association tasks and word category association tasks that are not described in detail here.
Visual Paradigms.
Activation of the visual cortex involves a large portion of the occipital cortex and has been investigated since the early days of fMRI research. Visual stimulation results in robust activation in the visual cortex, and visual stimuli typically involve different varieties of flashing checkerboard patterns. Such stimuli have been effective for both retinotopic mapping and assessing foveal vision.
Memory Paradigms.
Memory paradigms have not gained general acceptance for clinical fMRI, although much work has been performed at a research level using such paradigms. These can be difficult to perform and interpret, and susceptibility artifacts near the skull base can be a major limitation. Reliable memory lateralization with such paradigms has been difficult to accomplish. These are beyond the scope of this chapter and are not discussed in detail here.
Performing Clinical fMRI
The Setup
Because the magnitude of the BOLD signal is small, use of a high-field-strength magnet is essential; although 1.5 T is acceptable, the current standard is 3 T. At our institution, we perform fMRI studies on a 3-T scanner using a multichannel head coil. The stimulus paradigm is administered visually by displaying text or instructions via projector-mirror system or specialized goggles, or with auditory stimulus using specialized head phones especially for language/semantic tasks.
Pre-Scan Interview and Training
Reviewing recent prior imaging studies allows planning of the fMRI study and selection of paradigms tailored to the individual patient. Furthermore, pre-scan patient training is essential to familiarize patients with the tasks, assess their ability to adequately perform them, and alleviate anxiety.
Monitoring
Performance of foot, finger, or hand motor tasks can usually be monitored visually. Performance of some of the language tasks is monitored via patient responses/button presses, but performance of other covert language tasks cannot be directly monitored and must be assumed on the basis of the performance during the pre-scan training.
Real-time maps allow monitoring of task performance and also permit assessment of bulk head motion. These enable the neuroradiologist to make real-time informed decisions to repeat poor runs that are due to inadequate performance or excessive head motion that may have otherwise resulted in a nondiagnostic exam.
Postprocessing and Quality Control
After acquisition of data, several preprocessing steps are needed before the final statistical analysis and creation of activation maps.
As a part of postprocessing at our institution, we perform an extensive quality control analysis that includes assessment of patient head motion, functional-anatomic alignment, susceptibility artifacts, and data outlier volumes.
Analysis of fMRI Images
A variety of methods are available for analysis of fMRI. The most widely used is a regression analysis using a general linear model (GLM). The GLM can be written as Y = XB + U, where Y represents the fMRI data, X represents the design of the experiment, B represents the estimation parameters, and U represents the error term. For each voxel, a B is estimated that minimizes the error between the observed (dependent) data and a reference, or predictor. In clinical fMRI, a hemodynamic response function (HRF) convolved with the boxcar function of the task serves as the reference in a block-design paradigm. For cases with multiple tasks, each may be designated separately in the design matrix X, and corresponding beta coefficients calculated.
Following GLM fitting, an F statistic is calculated to determine how well the model explains a particular voxel’s time course. A high F statistic or low p value indicates that there is a high correlation between the model and the modulation of the voxel time course. A T-statistic is then calculated by contrasting the signal in one condition to a second (usually “control” or “rest”) condition.
A typical fMRI study at 3 T may have approximately 100,000 voxels. As a separate GLM analysis is performed for each voxel, this is also referred to as a massive univariate analysis. Given the large number of calculations performed, various methods have been employed to ensure that type I (false-positive) errors in activation are minimized. Ensuring specificity using multiple correction methods such as family-wise error (FEW), Bonferroni correction, false discovery rate (FDR), or cluster-based thresholding must be balanced with the need to avoid type II (false-negative) errors, which are more serious in clinical fMRI because surgical resection of areas labeled as no activation may result in significant morbidity.
There are many assumptions that must be met for fMRI analysis using GLM. Although most of these assumptions are beyond the scope of this book, one warrants discussion: fMRI GLM analysis relies on a canonical HRF to function as the basis for the reference. The HRF, however, may be variable across brain regions, across different age ranges, and even vary with specific stimulus conditions. Thus alternate methods have been investigated for model-free analysis of fMRI, including independent component analysis (ICA), which allows greater flexibility in discovering task-related and non–task-related signals without necessitating assumptions about their time courses.
Role of Streamlined Postprocessing Tools
Research-level BOLD fMRI postprocessing software is very accurate but relatively cumbersome, requiring significant experience in image processing, as well as computer programming, for generation of custom-made scripts for postprocessing. The main drawbacks in the use of such software include lack of U.S. Food and Drug Administration (FDA) approval and inability to efficiently export postprocessed images to picture archiving and communication systems (PACS) servers and neuronavigation systems. However, there are commercially available FDA-approved software packages now available that are more user friendly, allow for better image overlays on high-resolution anatomic images, and are compatible with both PACS servers and neuronavigation software. This has enabled import of functional images into the operating room, such that neurosurgeons can use the data in surgical planning. These packages also provide multiple options for statistical thresholding (e.g., cross-correlation, P value, T-statistic) and quality control analysis. At the same time, the research software allows for additional processing in situations where processing with the commercially available packages is inadequate.
Challenges and Limitations
BOLD fMRI is very sensitive to susceptibility and motion artifacts. Susceptibility artifacts can be especially problematic in patients who have had significant hemorrhage in the vicinity of the areas of interest or in those who have had prior surgery, with susceptibility artifacts from calvarial metallic implants/fixation devices. There may be situations where patients are not able to perform the tasks owing to lack of understanding, disability, or inability to cooperate for the exam.
BOLD fMRI is also affected by so-called venous artifacts because of intravascular BOLD effects. These changes in blood flow and oxygenation may propagate downstream in the brain vasculature and appear in larger draining veins distant from the active cortical areas. The venous location of the activation can, however, be confirmed by comparison of functional activation maps with high-resolution (contrast-enhanced) structural images.
Another problem associated with fMRI in the clinical setting is neurovascular uncoupling. As mentioned earlier, BOLD fMRI is an indirect measure of neuronal activity based upon the related hemodynamic changes. The absence of activation on BOLD fMRI does not imply the lack of neuronal activity. There are conditions under which the BOLD response may be disturbed, as with large vascular malformations or brain tumors with associated neovascularity, some low-grade tumors, or in situations with severe proximal arterial stenosis or strokes, which may give rise to false-negative activation.
Maps of cerebrovascular reactivity obtained using hypercapnia stimuli can be tools that provide an overall whole-brain view of the BOLD response. At our institution, we routinely use a breath-hold (BH) technique for this purpose. This BH technique involves short-duration BHs alternating with longer periods of normal self-paced relaxed breathing (we use a 16-second BH block with a normal breathing block of 40 seconds), repeated multiple times. Monitoring of task performance is performed using a standard respiratory belt. Postprocessing, however, needs to be performed with specific modeling to account for the differing durations of the BH and normal breathing blocks. The information obtained can be used to validate the ability of a perfusion bed to respond to a physiologic stimulus and the reliability of the fMRI mapping data.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree