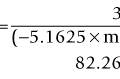Chapter 19 Future Considerations
Historical aspects
The field of ophthalmic ultrasonography essentially began in 1938 with the study of high intensity ultrasound effects on the eye by Zeiss.1,2 However, it was not until 1956 that the first ultrasonograph was published by Mundt and Hughes.3 The concept of a standardized ophthalmic ultrasonograph or standardized echography examination using A-mode was first introduced by Ossoinig in 1965 at the Eye Department of the University of Vienna.4 The following year, the International Society for Diagnostic Ophthalmic Ultrasound was founded.
Although B-mode ultrasound of the eye was first performed in 1958, it is the invention of the contact B-scan in 1972 that facilitated routine ophthalmic evaluation. The development of duplex Doppler ultrasound machines in the 1980s prompted diagnostic ophthalmic applications of this modality in the early 1990s.2 The availability of high frequency transducers led to the concept of ultrasound biomicroscopy, which was introduced by Pavlin in 1991.5 This and subsequent developments in ultrasound technology have greatly advanced the role of ophthalmic imaging in recent years. Some of the recent innovations and future considerations are discussed in the following sections.
High-frequency ultrasound and biomicroscopy
The use of micromachined ultrasound transducers has led to the development of clinically applicable high frequency transducers available for ophthalmologic ultrasonography in order to resolve small structures. The choroid, retina, and sclera can be readily distinguished at 20 MHz.6 Thus, high frequency ultrasound is superior to computerized tomography (CT) or magnetic resonance imaging (MRI) for detecting lesions that are 2 mm or less in thickness.7 While higher frequencies improve spatial resolution, there is a trade-off with depth of penetration. Nevertheless, probes with frequencies of 50 MHz and higher have been designed for ultrasound biomicroscopy.5 This technique generally uses B-mode ultrasonography with axial resolution in the order of 50 µm. The attenuation of the high-frequency ultrasound waves in biomicroscopy limits imaging to a depth of about 5 mm (Chapter 4).8
Nanotechnology applications in micromachining technologies enable fabrication of electrostatically driven membranes in the nanometer scale.9 Surface micromachined, capacitive ultrasonic transducers have been fabricated using complementary metal-oxide semiconductor (CMOS)-compatible processes. Novel transducer materials, such as lithium niobate and sol–gel composites, are capable of generating frequencies higher than 100 MHz.10
Doppler ultrasound
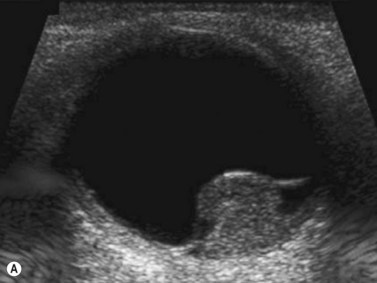
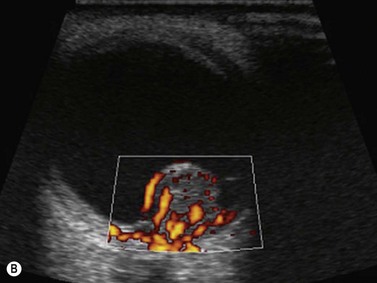
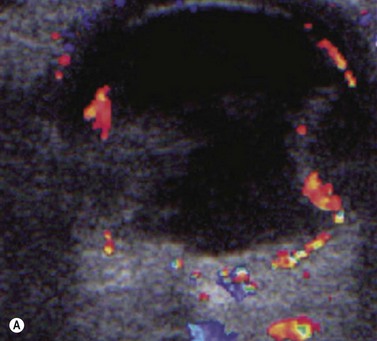
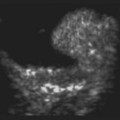
Doppler ultrasound is a technique that is used to evaluate blood flow, which can be depicted in color and in spectral tracings. Advances in Doppler ultrasound instrumentation permit detection of the slow-flow blood velocities in the ophthalmic artery and its branches, including the central retinal artery, the posterior ciliary artery, the lacrimal artery, as well as the superior ophthalmic vein, the vortex vein, and the central retinal vein (Chapter 5).11,12 Furthermore, Doppler spectral analysis can provide quantitative evaluation of blood flow velocity in these vessels. Thus, Doppler ultrasound plays a role in characterizing orbital tumors, carotid–cavernous sinus fistulas, central retinal artery occlusion, central retinal vein occlusion, giant cell arteritis, diabetic retinopathy, and ocular tumors (Figure 19.1).13 Power Doppler is superior to color Doppler technique for evaluation of the anatomy of orbital arteries and veins. Although sensitive to slow flow, the use of power Doppler in ophthalmic imaging is often limited due to artifacts.
Harmonic and superharmonic ultrasound
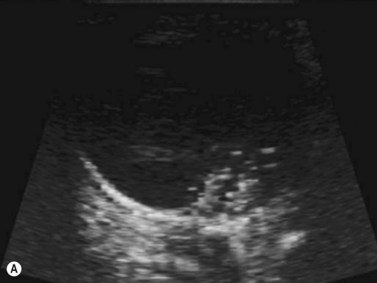
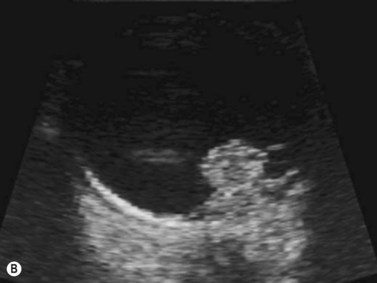
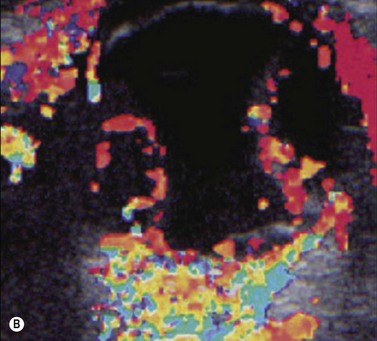
Harmonic imaging is based on the nonlinear interaction of the emitted ultrasound with the tissues, resulting in higher frequencies that return to the probe. This technique decreases artifacts and improves axial and lateral resolution. Imaging of first harmonics is widely used for many ultrasound applications. Superharmonic imaging is similar, but technically more challenging. Superharmonic imaging captures ultrasound beyond the second harmonic, yielding even greater contrast-to-tissue ratio. Detection of the transmission frequency up to the fifth harmonic is feasible, but necessitates a bandwidth greater than 130%. Phased array transducers with a wide dynamic range have been developed for this purpose. These transducers contain two different types of elements assembled in an interleaved pattern. The elements operate independently at different frequencies, allowing for separate transmission and reception modes.13–16 High-frequency contrast harmonic imaging of ophthalmic tumor perfusion has been reported (Figure 19.2).13–16
Contrast-enhanced ultrasound
Contrast-enhanced ultrasound (CEUS) is a technique that can be performed in real time following intravenous injection of microsphere or microbubble ultrasound contrast agents (UCA). Most UCA are composed of fluorocarbon or air and are stabilized by protein, lipid, or galactose-based substances. The half-life of UCA is several minutes. The imaging properties of UCA relate to the nonlinear interaction with the ultrasound waves, analogous to harmonic imaging (Chapter 5).
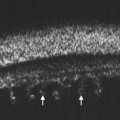
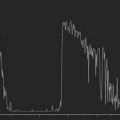
As compared to CT and MRI, CEUS is cost-effective, portable, produces no ionizing radiation, has no nephrotoxicity, and, most importantly, can provide comparable diagnostic information. Current applications of CEUS are for imaging visceral organs, abdominal aorta, genitourinary system, and breasts when standard ultrasound is inconclusive. In the realm of ophthalmic ultrasonography, UCA has been reported to slightly improve the detection of small vessels in uveal melanoma and helps differentiate a solid tumor from subretinal hemorrhage or effusion.17 However, CEUS does not distinguish normal vessels from tumoral vessels. CEUS has been combined with Doppler ultrasound to improve differentiation of retinal detachment from vitreous membrane (Figure 19.3).18