Multiparametric MR imaging is being used for prostate cancer diagnosis. However, many cancers are missed and the performance needs to improve before it can be used for population-level screening. We can expect standardization of multiparametric MR imaging and increased use of quantitative multiparametric MR imaging, which will lead to more reproducible results and improved interpretation. The development and integration of new acquisition techniques and use of artificial intelligence for image interpretation can lead to implementation of new clinical MR methods. These will lead to increased adoption of multiparametric MR imaging for prostate cancer diagnosis and for guiding intervention and follow-up.
Key points
- •
Multiparametric MR imaging is increasingly being used for prostate cancer diagnosis. However, the performance of MR imaging needs to improve before it can be used for population-level screening.
- •
Despite attempts to standardize the reporting and interpretation of prostate MR imaging, adherence to the recommended standards remains a problem.
- •
The current state of prostate multiparametric MR imaging is based on subjective radiologist assessment. However, there is increasing evidence that quantitative measurements can improve the diagnosis of prostate cancer.
- •
The most widely accepted MR parameters used for prostate cancer detection clinically provide little information regarding the underlying histology and, therefore, the use of structural models may be useful in the future.
- •
Since, CAD is more sensitive to subtle changes, their role might be even more critical for focal therapy planning, especially to determine the optimal treatment option by differentiating between clinically significant and non-significant cancers and in determining the cancer tumor volume to reduce the number of recurrences due to incomplete ablation of cancer tissue.
Introduction
Prostate cancer is the most common noncutaneous cancer and among the leading cause of death among men in the United States. Screening for elevated prostate-specific antigen has facilitated diagnosis of early stage clinical and pathologic stages of prostate cancer. However, prostate-specific antigen testing has low sensitivity in prostate cancer detection. Transrectal ultrasound-guided biopsies prompted by elevated serum prostate-specific antigen levels are also limited in the accurate understanding of the aggressiveness of the disease. Although histology remains the reference standard for prostate cancer diagnosis, a transrectal ultrasound-guided biopsy samples a very small part of the prostate (approximately 1%) and often miss cancers, especially those located in the anterior of the prostate. Additionally, the Gleason score of the cancer diagnosed by transrectal ultrasound-guided biopsy is upgraded for an estimated 30% of patients undergoing repeat biopsy or radical prostatectomy. Therefore, understanding the aggressiveness of cancer (distinguishing low-risk tumors from potentially life-threatening tumors) cannot be reliably determined, which causes many patients with low-risk tumors elect to undergo unnecessary surgery or radiation therapy. These limitations emphasize the need for a noninvasive and accurate evaluation method for the entire prostate for the detection, staging, and risk stratification of prostate cancer, which will lead to a better choice of treatment and decrease the overtreatment of indolent disease.
Multiparametric MR imaging is increasingly used for prostate cancer diagnosis and guiding biopsies. It has a high negative predictive value and good sensitivity and specificity in prostate cancer diagnosis. It also has the potential to provide reliable information about the cancer grade, location, and volume for the selection of optimum therapy. Multiparametric MR imaging acquisition protocols and interpretation guidelines were recently recommended by American College of Radiology and European Society of Urogenital Radiology in the consensus guidelines entitled Prostate Imaging – Reporting and Data System (PI-RADS v2). Recent studies such as the PROMIS and PRECISION trials support the concept of targeted biopsies, using multiparametric MR imaging for target selection. This approach is found to be more cost effective and can lead to increased acceptance of multiparametric MR imaging for prostate cancer screening. Despite these promising results, 10% to 20% of clinically significant cancer can be missed on multiparametric MR imaging. Benign features such as prostatitis, prostatic hyperplasia, and atropy can mimic cancer, whereas cancer detection in the transition zone (site of approximately 30% of cancers) remains limited. In addition, there is high interobserver variability in the interpretation of multiparametric MR imaging among radiologists, and the lack of image acquisition standards along with a lack of quality control measures for these imaging sequences limits the reproducibility of the performance of prostate MR imaging studies. Therefore, the performance of MR imaging still needs to improve before it can be used for the screening of large populations. In this article, we briefly summarize the current state of prostate multiparametric MR imaging and its drawbacks, focusing on the future perspectives in multiparametric prostate MR imaging.
Current state of multiparametric MR imaging of the prostate
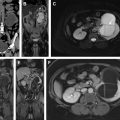
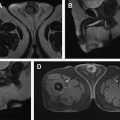
Prostate multiparametric MR imaging consists of T2-weighted imaging, diffusion-weighted imaging (DWI), precontrast T1-weighted imaging, and dynamic contrasted-enhanced (DCE) MR imaging. Multiparametric MR imaging is highly sensitive in the detection of prostate cancer compared with any individual sequence. A study reported that individually T2-weighted MR imaging (58%), DWI (53%), and DCE-MR imaging (38%) sequences demonstrate lower sensitivity compared with multiparametric MR imaging (85%). The reported positive and negative predictive value for prostate cancer detection using multiparametric MR imaging is also much higher than that using either of these imaging sequences individually.
T2-Weighted Imaging
T2-weighted imaging allows excellent soft tissue contrast along with good spatial resolution and a high signal-to-noise ratio. It provides good visualization of the zonal anatomy, seminal vesicles, and neurovascular bundle. High-resolution T2-weighted imaging with 2-dimensional turbo/fast spin echo or 3-dimensional spin echo sequences with in plane resolution of less than or equal to 0.4 to 0.7 mm and a slice thickness of 3 mm or less is recommended. Images are acquired in the axial, sagittal, and coronal planes, whereas DWI, T1-weighted, and DCE-MR imaging are obtained in the same planes as axial T2-weighted imaging. Prostate cancer is hypointense on T2-weighted images compared with benign tissue and quantitative T2 values are significantly lower in prostate cancer compared with benign prostate tissue.
Diffusion-Weighted Imaging
DWI provides a signal sensitive to water movement and provides information about the tissue structure and density. A spin echo, echo planar imaging pulse sequence with a plane resolution of greater than 2.5 mm and slice thickness of 4 mm or less is generally used clinically. Images with at least 2 b -values are required to acquire an apparent diffusion coefficient (ADC) map using a monoexponential signal decay model. A lower b -value of 50 to 100 s/mm 2 and higher 800 to 1500 s/mm 2 is generally used. On high b -value (approximately 1500 s/mm 2 images) increased signal is seen in prostate cancer owing to reduced diffusivity. ADC is a measure of the magnitude of the diffusivity of water molecules and is used extensively clinically for the detection of cancer. ADC in cancer tissue is lower than in normal tissue and there is an inverse relation between ADC value and cancer Gleason grade. A meta-analysis of 10 studies reported that the combined use of diffusion MR imaging, specifically ADC with traditional T2-weighted images demonstrated higher sensitivity (76%) and specificity (82%) compared with T2-weighted images alone. In addition, tumor volumes estimated from DWI have been shown to demonstrate better correlation with histologic volume either than T2-weighted or DCE-MR imaging.
T1-Weighted Imaging
Precontrast T1-weighted images are taken over a large field of view using spin or gradient echo pulse sequence either with or without fat suppression to observe postbiopsy changes. T1 hyperintensity in the prostate is usually due to hemorrhage in the prostate after biopsy.
Dynamic Contrasted-Enhanced MR Imaging
DCE-MR imaging involves the acquisition of serial T1-weighted images (fast/spoiled gradient echo sequence with fat suppression) of the prostate before and after the bolus injection of a chelated gadolinium molecule. Cancers show early focal signal enhancement owing to increased vascularity or angiogenesis. Increased capillary permeability leads to higher uptake of contrast agent that shortens T1 relaxation time and therefore shows up as hyperintense region with respect to surrounding tissue. A temporal resolution of less than 10 seconds is recommended because the use of higher temporal resolution leads to increased diagnostic performance.
A representative example of prostate cancer seen on multiparametric MR imaging currently used clinically is shown in Fig. 1 .

Future of multiparametric prostate MR imaging
Standardization of Multiparametric MR Imaging
Prostate multiparametric MR imaging is continuously evolving. Initial consensus guidelines for prostate multiparametric MR imaging, the PI-RADS v1, was introduced by the European Society of Urogenital Radiology in 2012. More recently, updated consensus guidelines by the American College of Radiology and European Society of Urogenital Radiology know as PI-RADS v2 were introduced in 2015. MR spectroscopy, which was an integral part of PI-RADS v1, is not used as a sequence in prostate multiparametric MR imaging any more. T2-weighted imaging and DWI are now the dominant sequence for PI-RADS scoring in the transition and peripheral zones, respectively, whereas the role of DCE-MR imaging is secondary. Despite attempts to standardize the reporting and interpretation of prostate MR imaging by PI-RADS, a recent investigation found that adherence to the recommended standards remains a problem.
Moreover, there is a lack of consensus on the details of the imaging sequence parameters. Standardized imaging is particularly important for quantitative image analysis and multicenter trails. For example, it has been shown that the use of different b -values and its range and diffusion time affects ADC calculation and subsequent diagnostic performance among radiologists. Most clinical scanners do not specify important diffusion parameters such as gradient strength and gradient time and how specific diffusion weighting is done, so different scanners using the same b -value might have different ADC values for the same tissue. Similarly, diagnostic results vary for T2-weighted imaging when different echo time are used. The standardization is even more critical for DCE-MR imaging, where the variation for image acquisition is even greater. It has been shown that high temporal resolution tends to improve the performance of DCE-MR imaging in a prostate cancer diagnosis and therefore PI-RADS v2 recommends a temporal resolution of less than 10 seconds. In addition, the use of different contrast agents with varying relaxivity affects the signal and image analysis.
There is a lack of quality control measures for these imaging sequences. In the future, clear standards and certifications need to be established. Imaging quality testing with phantom and patient imaging needs to be implemented such that prostate multiparametric MR imaging adheres to image quality assessment standards.
The variability in the imaging protocols and the lack of quality control measures limit the reproducibility of the performance of prostate MR imaging studies. It has been shown that the introduction of PI-RADS v2 provided only moderately reproducible MR imaging results for the detection of clinically significant cancer. In light of these variations that affect multiparametric MR imaging interpretation, it might be judicious in the future for recommending the standardization of imaging protocols for more reproducible prostate multiparametric MR imaging results. The training and certification of radiologists interpreting prostate multiparametric MR imaging needs to be standardized to produce reproducible results.
Hardware Improvements
There have been substantial recent advances in MR hardware. More clinical centers are replacing their 1.5 T with 3 T MR systems, and some 7 T systems (primarily for research at this point) have recently been available clinically. The main advantages of increased MR magnetic field strength are increased signal-to-noise ratio, which improves spatial resolution; decreased acquisition time; and increased temporal resolution for DCE-MR imaging. Studies have found that increased signal-to-noise ratio may lead to improvement in prostate cancer localization. However, the tissue T1 relaxation times increase with high fields and may be mitigated by the loss in contrast enhancement in T1-weighted images owing to improved background suppression.
Furthermore, the use of an endorectal coil in addition to a surface coil can be used to improve image quality. Barium sulfate suspension or perfluorocarbon fluid can be used to inflate the endorectal coil to squeeze air out to reduce susceptibility and motion artifacts. Endorectal coil increases the sensitivity and positive predictive value for prostate cancer detection over not using an endorectal coil. The use of dedicated coils for prostate imaging and phase array coil with more receiver channels can lead to major improvements in signal-to-noise ratio as well as higher acceleration for parallel imaging. Improved gradients coils can improve multiparametric MR imaging with DWI with higher b -values achievable. Newly designed coils using digital parallel transit/receive radiofrequency technology, compressed sensing, and so on have the potential to improve the performance of multiparametric MR imaging in the future.
Quantitative Multiparametric MR Imaging
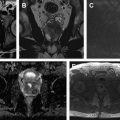
The current state of prostate multiparametric MR imaging is based on subjective assessment by the radiologist. However, there is increasing evidence that quantitative measurements can improve the diagnosis of prostate cancer. ADC remains the backbone of prostate cancer diagnosis, with ADC values for prostate cancer being significantly lower than those for normal tissue. In addition, numerous studies have shown a negative correlation between ADC values and Gleason score; therefore, ADC values can be used as a predictor of tumor aggressiveness. Although monoexponential models like ADC and T2 are used often, it has been shown that diffusion and T2-weighted signal are not monoexponential. Numerous models such as biexponential, intravoxel incoherent motion, kurtosis, stretched exponential signal models, and so on have been proposed. Although quantitative T2 numbers are not used clinically, they have been shown to be significantly lower in prostate cancer compared with normal tissue. A recent study has demonstrated that T2 maps have similar sensitivity but greater positive predictive value for detecting prostate lesions compared with standard T2-weighted images. Quantitative and semiquantitative DCE-MR imaging metrics measured using Tofts’ or empirical mathematical model have been shown to be effective in prostate cancer diagnosis. A representative example of prostate cancer seen on quantitative multiparametric MR imaging derived maps is shown in Fig. 2 .

However, in addition to the absence of standard technique, the integration of quantitative multiparametric MR imaging is also challenging owing to the additional postprocessing and increased time involved. Despite these challenges, quantitative multiparametric MR imaging can lead to a major improvement over the subjective nature of qualitative assessment of prostate MR imaging. Therefore, with powerful computing capabilities, it will be possible to integrate quantitative analysis to prostate multiparametric MR imaging.
New Acquisition Methods
Another direction of numerous studies has been faster acquisition and reconstruction methods. The use of abbreviated protocol has also been investigated. MR spectroscopy was a recommended protocol earlier; however, with the introduction of the PI-RADS v2 guidelines, MR spectroscopy is not used any more. Few studies have shown that biparametric MR imaging, using only T2-weighted and DWI, offers similar diagnostic accuracy for prostate cancer detection in addition to a decrease in imaging time compared with that of conventional multiparametric MR imaging including DCE-MR imaging. Other accelerated acquisition and reconstruction methods that have been investigated, including k-t-T2, high temporal resolution or ultrafast DCE, sensitivity encoding, and spiral or radial imaging. Another technique that has garnered a lot of attention is MR fingerprinting. Although it allows rapid, simultaneous generation of quantitative maps of multiple physical properties such as T1 and T2, it has inherent drawbacks for prostate cancer diagnosis because it only provides similar images to standard multiparametric MR imaging.
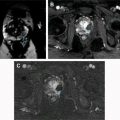
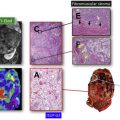
The most widely accepted MR parameters (eg, ADC, T2 and T1 values) used for prostate cancer detection clinically provide little information regarding the underlying complex microstructure or histology (Gleason grading), which remains the reference standard. Tissue composition in prostate cancer is different from normal prostate. Recently proposed prostate tissue structural models include the VERDICT model and Luminal volume imaging, which use increased cellularity and reduced luminal fluid as the primary markers for cancer detection. Hybrid multidimensional MR imaging was recently proposed, which exploits the distinct MR properties of tissue components, to estimate fractional volumes of the prostate gland components. Prostate cancers show increased epithelium and reduced lumen and stroma volume compared with benign tissue, and these changes correlate with Gleason grade better than conventional ADC and T2. A representative example of tissue composition map estimated noninvasively through hybrid multidimensional MR imaging and its usefulness in prostate cancer detection is shown in Fig. 3 .

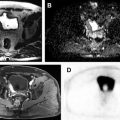
There have been recent debates regarding the use of gadolinium-based contrast agents owing to the concerns with the deposition of gadolinium in tissue, especially in the brain, liver, bone, and skin. Although there are no clinical consequences of gadolinium deposition, there are calls for improved and different approaches despite the diagnostic benefits of contrast agents. Preliminary results suggest that DCE-MR imaging with low gadolinium dose contrast agent or high relaxivity agent can distinguish prostate cancer from benign prostate tissue as effectively as the standard dose. A representative example showing a diagnosis of prostate cancer using low quantities (15% of standard clinical dose) of a high relaxivity contrast agent is shown in Fig. 4 . Additionally, newly synthesized contrast agents such as iron and vanadium based contrast agents are being investigated and will possibly be used in future multiparametric MR imaging protocols.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree