Chapter Outline
Clinical Presentation and Evaluation
Diagnostic and Therapeutic Procedures
Upper Gastrointestinal Hemorrhage
Lower Gastrointestinal Hemorrhage
Gastrointestinal hemorrhage is one of the most common and challenging clinical problems encountered by the gastroenterologist. Approximately 400,000 patients with signs and symptoms of gastrointestinal hemorrhage seek medical attention each year in the United States. Fortunately, in the majority of cases, gastrointestinal hemorrhage resolves spontaneously. However, in 25% of patients with recurrent or persistent bleeding, morbidity and mortality are significant. In these patients, rapid and accurate diagnosis and treatment of the bleeding source are necessary to prevent death and to limit morbidity. In many patients, bleeding may be chronic and intermittent, and patients may present with subtle signs of blood loss, such as anemia. Patients with chronic gastrointestinal blood loss have a lower mortality rate compared with patients with acute bleeding, but the toll on quality of life and the cost of care due to repeated hospitalizations and diagnostic procedures are substantial.
The classification of gastrointestinal hemorrhage is based on the severity of bleeding and the site of bleeding within the gastrointestinal tract. Patients experiencing gastrointestinal blood loss who exhibit signs of hemodynamic instability require rapid resuscitation and prompt identification and treatment of the bleeding site in an inpatient setting. On the other hand, hemodynamically stable patients with signs of chronic blood loss may be managed as outpatients.
On the basis of endoscopic accessibility, gastrointestinal tract bleeding is also classified by the segment of the gastrointestinal tract in which the bleeding occurs. Upper gastrointestinal tract bleeding is defined as bleeding that originates from a lesion proximal to the ligament of Treitz, that is, the segment of bowel routinely accessible by upper endoscopy. Midgut bleeding occurs between the ampulla of Vater and the terminal ileum (accessible by deep endoscopy); and lower gastrointestinal tract bleeding occurs distal to the terminal ileum (accessible by colonoscopy). The approximate frequency of bleeding in each of these segments is 75% to 80%, 5% to 10%, and 20% to 25%, respectively.
The remainder of this chapter discusses an approach to the diagnosis and treatment of patients with gastrointestinal hemorrhage with emphasis on the imaging tools used to detect the nature and location of bleeding and the common methods used in the treatment of gastrointestinal hemorrhage.
Etiology
The list of gastrointestinal bleeding sources is extensive ( Table 125-1 ). Most disorders are more common in specific segments of the gastrointestinal tract, and the more common conditions responsible for bleeding are discussed in the appropriate sections.
| Upper Tract Hemorrhage |
|
| Upper and Lower Tract Hemorrhage |
|
| LOWER TRACT HEMORRHAGE |
|
Past medical history often provides helpful clues to the source of gastrointestinal bleeding. Up to 60% of patients with a history of upper gastrointestinal hemorrhage bleed from the same lesion. Comorbid conditions may also suggest a potential cause of bleeding:
- •
Liver disease or alcohol abuse: varices
- •
Renal disease, aortic stenosis, or Osler-Weber-Rendu disease: angiodysplasia
- •
Abdominal aortic aneurysm or aortic graft: aortoenteric fistula
- •
Helicobacter pylori infection: peptic ulcer disease
- •
Smoking, alcohol abuse, H. pylori infection: malignant disease
- •
Gastroenteric anastomosis: marginal ulcers
- •
Chronic diarrhea and abdominal pain: inflammatory bowel disease
Clinical Presentation and Evaluation
Gastrointestinal hemorrhage has five clinical presentations: hematemesis, which is bloody vomitus that may be fresh and bright red or older with a coffee-ground appearance; melena, which is black, shiny, sticky, foul-smelling fecal matter that results from the degradation of blood in the gut; hematochezia, which is the passage of bright red or maroon blood, bloody diarrhea, or blood mixed with formed stool; occult blood, found only by testing of the stool with a chemical reagent; and symptoms of blood loss, such as dyspnea, dizziness, or shock (in massive bleeding) or iron deficiency anemia (in chronic bleeding).
A major goal in the diagnosis of gastrointestinal hemorrhage is differentiation of upper from more distal gastrointestinal tract sources of bleeding. Proximal lesions tend to cause hematemesis or melena, whereas distal lesions more commonly produce hematochezia. Hematochezia stemming from an upper gastrointestinal source usually reflects a massive hemorrhage, which, if it is associated with a bloody nasogastric aspirate, has a mortality of nearly 30%. Hematemesis almost invariably localizes the source of hemorrhage proximal to the ligament of Treitz. However, up to 40% to 50% of patients with upper gastrointestinal bleeding do not experience hematemesis. About 20% of patients with bleeding ulcers present with melena, 30% with hematemesis, 50% with both, and as many as 5% with hematochezia. Melena can result when as little as 50 mL of blood is instilled into the upper gastrointestinal tract; the instillation of 1000 mL or more leads to hematochezia.
Aspiration of gastric contents with a nasogastric tube may reveal blood, which is diagnostic of an upper gastrointestinal bleeding source. The absence of blood, however, does not entirely exclude the possibility of an upper gastrointestinal hemorrhage because the bleeding might have ceased before passage of the tube or might have occurred distal to a competent pyloric sphincter. The presence of bile in the gastric aspirate indicates that the bowel distal to the pylorus has been sampled. Nasogastric lavage may help clear the stomach of blood clots and debris before endoscopy; however, there is little evidence to suggest that lavage helps stop bleeding.
Diagnostic and Therapeutic Procedures
Endoscopy
Upper endoscopy and colonoscopy are the most important tools for evaluation of patients with gastrointestinal bleeding because they permit direct visualization of the bowel lumen and provide a method for rapid diagnosis and treatment of abnormalities within the upper gastrointestinal tract and within the colon and terminal ileum. Endoscopy can determine the cause of bleeding with a 90% to 95% accuracy while affording access for therapeutic procedures and providing valuable prognostic information.
Whereas endoscopy remains the cornerstone of diagnosis and therapy for gastrointestinal bleeding originating in the upper gastrointestinal tract and colon, until recently the small bowel has been inaccessible. Recent advances in endoscopic techniques, including capsule endoscopy and deep enteroscopy, have extended the usefulness of endoscopy to the entire gastrointestinal tract, including the small bowel.
Capsule Endoscopy
Capsule endoscopy (CE) was introduced in 2000 and enables visualization of the entire gastrointestinal tract, including the small bowel, which until then was virtually inaccessible with conventional endoscopic techniques. This technique involves the oral or endoscopic introduction of a small capsule equipped with a light source, lens, battery, radiofrequency transmitter, and antenna into the stomach or duodenum. The capsule acquires images of the gastrointestinal tract at two frames per second and transmits data to a data recorder worn on the patient’s waist. The images are then downloaded and viewed on a computer. CE allows visualization of the entire small bowel in 79% to 90% of patients.
CE does have several disadvantages. Trivial findings are commonly detected and occur in up to 23% of healthy patients undergoing CE. In addition, because the device is purely diagnostic, additional therapeutic procedures may be indicated if significant abnormalities are found. The most serious disadvantage of CE is capsule retention in patients with a stricture or bowel obstruction, sometimes requiring surgical removal. This is more common in patients with Crohn’s disease and small bowel tumors. Patency capsules are useful for minimizing the risk of retention by selecting those patients who may safely undergo CE.
Deep Enteroscopy
These techniques, used primarily to evaluate the small bowel, include double-balloon enteroscopy (DBE), single-balloon enteroscopy, and spiral enteroscopy. These techniques offer an advantage over CE because they not only permit visualization of the entire small bowel but also allow therapeutic interventions. These advanced techniques require special technical skill and have associated complications, such as bleeding and bowel perforation; therefore, they are usually performed only after a positive finding on CE or other imaging studies suggesting a small bowel abnormality.
Double- and single-balloon enteroscopes consist of an enteroscope and an overtube with an inflatable balloon. Double-balloon enteroscopes also have a balloon attached to the end of the enteroscope. Advancement through the small bowel is achieved by a series of push-pull maneuvers in which the balloon is alternately inflated and deflated as the enteroscope and overtube are advanced as the small bowel is shortened like the bellows of an accordion. These procedures can be performed by an antegrade or retrograde approach. The antegrade approach is selected for suspected lesions in the proximal 75%; the retrograde approach is selected for distal lesions. The choice of approach is based on CE or other imaging findings. The success rate for examination of the entire small bowel ranges from 16% to 86%. The complication rate for diagnostic DBE is 0.8%; for therapeutic procedures, it is 4%.
Spiral enteroscopy is a new technique using a spiral overtube, which has a 21-cm raised helix at the distal end. The overtube is placed over the enteroscope, and the paired device is advanced by rotation in a clockwise fashion until the farthest extent of the small bowel is reached. The enteroscope is then advanced alone, followed by rotation of the spiral overtube. This device permits significantly shorter examination time and has a complication rate similar to that of DBE.
Multidetector Computed Tomography
The utility of multidetector computed tomography (MDCT) in the diagnosis of gastrointestinal hemorrhage has become increasingly accepted. In a meta-analysis of 22 studies involving 672 patients with acute episodes of gastrointestinal bleeding, CT angiography achieved a sensitivity of 85% and specificity of 92% in detecting active bleeding. MDCT techniques also appear useful in evaluating the source of bleeding in hemodynamically stable patients. In a prospective study of outpatients with obscure gastrointestinal bleeding, multiphase CT enterography (CTE) identified a bleeding source in 14 of 16 patients.
In the majority of reports, the method used for evaluation of gastrointestinal bleeding is a modification of CTE technique (see Chapter 38 ). The patient drinks a large volume of low-concentration barium solution (e.g., VoLumen) to produce bowel distention. Water can be substituted, but it produces suboptimal bowel distention because of rapid intestinal absorption. Both agents are of neutral density to permit optimal bowel wall visualization. The presence of positive contrast material within the bowel, as is common with most CT scan studies, obscures the bowel wall and may mask subtle vascular lesions and prevent the identification of active bleeding.
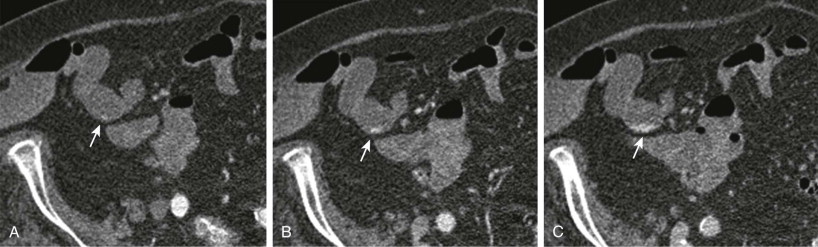
A variety of scan techniques for evaluation of gastrointestinal bleeding have been reported. These usually involve two- or three-phase acquisitions (e.g., arterial, portal venous, delayed) after intravenous administration of contrast material, sometimes combined with a preliminary noncontrast scan. Obviously, the greater the number of acquisitions, the higher the patient’s radiation dose. However, a greater number of acquisitions may improve detection of a bleeding source. The evolving appearance of active bleeding producing intraluminal accumulation of contrast material is best appreciated over time with multiple scan acquisitions ( Fig. 125-1 ). Also, some lesions have characteristic enhancement patterns that may permit a specific diagnosis and therefore encourage timely management. In addition, vascular lesions may be visible only transiently, and abnormalities may go undetected if the acquisition is too early or too late. The choice of technique depends on the patient’s age and clinical history. In our practice, we prefer to use three-phase acquisition for the evaluation of the majority of patients with obscure gastrointestinal bleeding.
Nuclear Scintigraphy
Radionuclide imaging in the evaluation of gastrointestinal hemorrhage can be classified into direct and indirect methods. Direct nuclear scintigraphic methods use either technetium Tc 99m sulfur colloid or the preferred 99m Tc-labeled red blood cell (RBC). Indirect evaluation of gastrointestinal blood loss relates to the use of 99m Tc-pertechnetate scintigraphy in suspected Meckel’s diverticulum, which is of particular value in younger patients.
99m Tc-sulfur colloid studies can be useful in demonstrating rapid gastrointestinal blood loss, but with a short intravascular residence time (half-life of 2-3 minutes) and increasing uptake in the bone marrow, liver, and spleen, it has limited usefulness in gastrointestinal blood loss, as active bleeding is almost always intermittent. This allows a limited imaging window of approximately 15 minutes to demonstrate the bleeding site. It is also an “off-label” indication for this radionuclide tracer.
99m Tc-labeled RBC scintigraphy is considered a sensitive noninvasive method for detection of gastrointestinal blood loss, with sensitivity ranging from 40% to 90%. In the animal model, it can detect blood loss rates on the order of 0.1 mL/min compared with 0.5 to 1.0 mL/min with contrast angiography. The examination requires little preparation of the patient, has relatively low radiation exposure (0.3 rad/20 mCi), and can usually cover more than 12 hours of intermittent imaging. Sensitive imaging is predicated on high and durable RBC labeling efficiency, on the order of 95% by postlabeling quality control. The highest labeling efficiency is by the in vitro labeling methodology with use of a commercial kit such as UltraTag (Mallinckrodt, St. Louis, MO). The labeling procedure takes 20 to 30 minutes, and the labeled RBCs are reinjected, acquiring anterior abdominal dynamic images under a large field of view gamma camera. Typically, 60 to 90 minutes of initial imaging is standard, with subsequent delayed images if initial images are normal, as dictated by the patient’s clinical status.
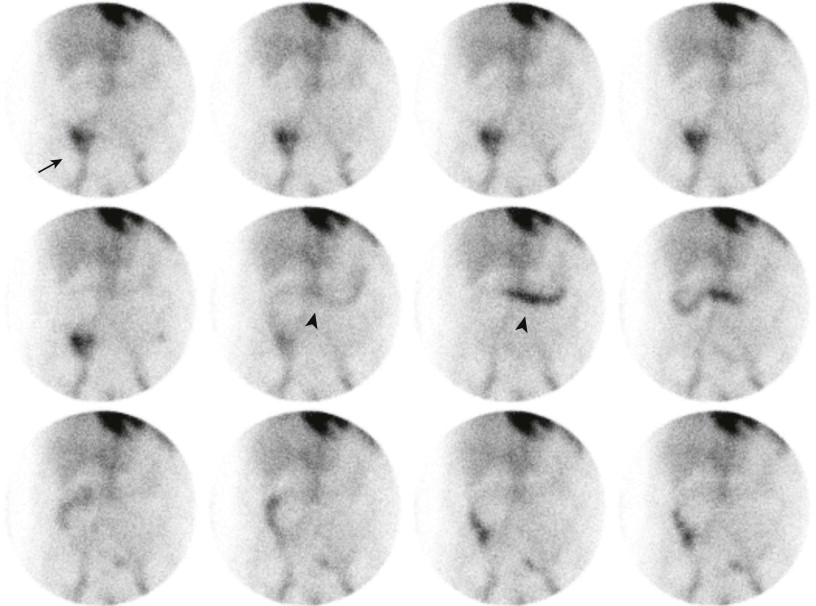
Image interpretation relies on viewing extravasation of the tracer into the bowel with subsequent peristaltic movement through the bowel ( Fig. 125-2 ). The configuration, location, and movement of the tracer over time determine whether the gastrointestinal bleeding site is from the small or large bowel, noting that both antegrade and less commonly retrograde tracer movement can occur.
Imaging pitfalls can be due to free technetium within the stomach and renal system. This may be seen with a poor 99m Tc-RBC label, unusual or prominent blood pool foci (such as abdominal aortic aneurysm), esophageal varices, penile activity, and vascular grafts as well as with miscellaneous items such as accessory splenic tissue and hemangiomas.
Angiography
Persistent or recurrent bleeding occurs in 7% to 16% of patients with upper gastrointestinal bleeding and in up to 25% of patients with lower gastrointestinal bleeding. Such patients may require angiographic intervention to locate or to treat the source of bleeding.
In most cases, angiographic control of gastrointestinal bleeding requires that the site of bleeding first be identified. Angiographic detection of active bleeding requires a minimum bleeding rate of 0.5 mL/min. Because isotope-labeled RBC scans can detect lower bleeding rates (0.1 mm/min), a positive nuclear study may predict a positive angiographic examination.
Therapeutic angiographic intervention has a wide spectrum of applications, from embolization of arterial lesions, to direct infusion of vasopressin, to performing a transjugular intrahepatic portal systemic shunt (TIPS). Intra-arterial vasopressin causes generalized vasoconstriction by a direct action on vessel walls, thereby decreasing perfusion pressure to all stable clot formation. Unfortunately, rebleeding is common after cessation of the infusion. As a potent peripheral vasoconstrictor, vasopressin must be used with caution in patients with coronary artery disease, congestive cardiomyopathy, severe hypertension, and peripheral vascular disease.
Therapeutic embolization causes mechanical occlusion of the blood supply to the bleeding site. The embolic agents used can produce temporary (0-21 days) or permanent vessel occlusion. The embolic agent is delivered through an end-hole catheter into the target vessel. Complications occur in 5% to 9%, with ischemia and infarction being the most common. The use of superselective catheters has decreased complications related to ischemia. The use of angiography in specific cases of gastrointestinal bleeding is discussed in the following sections.
Barium Studies
Barium studies have no significant role to play in assessing the patient with massive gastrointestinal hemorrhage. Barium in the bowel lumen interferes with endoscopic studies, diagnostic MDCT, and diagnostic and therapeutic angiography. In patients with anemia and occult gastrointestinal bleeding, barium studies have been replaced by upper gastrointestinal endoscopy, colonoscopy, CE, and MDCT.
Upper Gastrointestinal Hemorrhage
Upper gastrointestinal tract bleeding constitutes 75% to 80% of all cases of acute gastrointestinal tract bleeding. The incidence has decreased significantly in recent times; however, the mortality rate from acute nonvariceal upper gastrointestinal tract bleeding has decreased only minimally in the past 50 years, ranging from 2.5% to 10%. This lack of change in mortality rate likely is related to the increasing age of patients who present with upper gastrointestinal bleeding and an increase in associated comorbid conditions.
Peptic ulcers are the most common source of upper gastrointestinal bleeding, accounting for 20% to 40% of cases. Other major causes are gastric erosions (15%-25% of cases), bleeding varices (5%-30%), and Mallory-Weiss tears (5%-15%). The use of aspirin or nonsteroidal anti-inflammatory drugs is prevalent in 45% to 60% of all cases of acute upper gastrointestinal bleeding. Moreover, the risk of upper gastrointestinal bleeding is increased in patients who take as few as one “baby aspirin” (81 mg) per day.
The initial evaluation of a patient with upper gastrointestinal bleeding should focus on assessment of hemodynamic status and comorbid conditions. Factors associated with severe upper gastrointestinal bleeding include red blood from nasogastric lavage, tachycardia, and hemoglobin level below 8 g/dL.
As in all cases of gastrointestinal bleeding, consideration must be given to comorbid illnesses that predispose patients to hypoxia (coronary artery disease, chronic obstructive pulmonary disease), volume overload (congestive heart failure, renal failure), bleeding (coagulopathies, thrombocytopenia, liver disease), and aspiration (dementia, hepatic encephalopathy) in choosing diagnostic and therapeutic procedures.
Expedited use of upper endoscopy is the diagnostic procedure of choice for acute upper gastrointestinal bleeding. Its reported sensitivity and specificity for the diagnosis of upper gastrointestinal bleeding are 92% to 98% and 30% to 100%, respectively. In addition, once bleeding is identified, endoscopic treatment can achieve hemostasis and prevent recurrent bleeding in most patients.
Urgent endoscopy, however, cannot be advocated for all patients with acute upper gastrointestinal hemorrhage. Several prospective, randomized studies published in the early 1980s failed to demonstrate a decrease in morbidity or mortality rates associated with endoscopy in diagnosis of upper gastrointestinal hemorrhage.
Upper gastrointestinal endoscopy, however, should be performed as soon as possible in high-risk individuals: alcoholic patients who may be bleeding from a variety of sources; patients with suspected aortoenteric fistula; those with a quantitatively large volume of blood loss; those with suspected active hemorrhage; and those experiencing or unlikely to tolerate a recurrent hemorrhagic episode, including those who object to blood transfusions on religious grounds. Patients with minor bleeding (i.e., without tachycardia, hypotension, or decreased hematocrit) can wait until the next day for elective endoscopy. Younger, otherwise healthy patients with trivial bleeding can be discharged without diagnostic testing with outpatient follow-up evaluation.
Peptic Ulcers
Bleeding from gastric or duodenal ulcers remains the most common cause of upper gastrointestinal tract hemorrhage. The most common risk factors for peptic ulcer disease are Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs, physiologic stress, and excess gastric acid (e.g., Zollinger-Ellison syndrome).
The approach to a patient who has bled from peptic ulcer disease is determined at the time of endoscopy. There are many options for endoscopic therapy. Thermal-coaptive coagulation (an endoscopic technique used to ablate bleeding ulcers in which two vessel walls are compressed and “fried”) involves the placement of the coagulating probe directly on the bleeding vessel. This is uniformly effective for vessels up to 2 mm in diameter with the heater probe. Injection therapy results in short-term tamponade and vasospasm and can be induced with the liberal use of epinephrine (1 : 10,000). Vasodestruction is long term and can be induced by sclerosants or alcohol (total injection volume not to exceed 2 mL). Endoscopic clipping has not been shown to be any more effective than thermal therapy ; however, it may have appeal for use in patients with coagulation disorders or in cases in which further coaptive coagulation may not be desirable.
Endoscopic therapy is indicated for patients with active arterial bleeding and those with a nonbleeding visible vessel (pigmented protuberance). An adherent clot is a predictor of rebleeding and can be managed with endoscopic therapy or high-dose proton pump inhibitor therapy (or both). All three endoscopic treatment options have been shown to have a relatively similar efficacy. However, epinephrine injection, followed by a more permanent form of treatment (coagulation, vasodestruction, or clipping), has been shown to be more effective than epinephrine therapy alone. Patients with a clean ulcer base (rebleeding rates, <5%) and a flat pigmented spot (rebleeding rates, 5%-10%) do not require endoscopic therapy and probably could be discharged soon after endoscopy. Deep ulcers may tend to expose larger vessels that may not be amenable to endoscopic coagulation. Deep ulcers in the stomach, particularly those in the upper body on the lesser curvature (left gastric artery), or posterior duodenal bulb (gastroduodenal artery) with nonbleeding visible vessels more than 2 to 3 mm in diameter should not be treated endoscopically. These patients may benefit from therapeutic embolization.
Rebleeding after endoscopic therapy occurs 20% to 30% of the time. Re-treatment for recurrent bleeding achieves long-term hemostasis in more than 70% of cases. The endoscopic appearance of the ulcer may predict its likelihood of rebleeding. An endoscopically visualized, actively bleeding ulcer carries an 80% chance of persistent or recurrent bleeding. A nonbleeding visible vessel is associated with a 50% chance of rebleeding.
If endoscopic therapy fails, angiographic embolization of the bleeding vessel is a preferable option to surgical intervention. Vasopressin is effective at controlling gastric bleeding (70%). Bleeding duodenal ulcers are relatively unresponsive to vasopressin infusion because of the dual blood supply to the duodenum; the inflammatory reaction produced by the penetrating ulcer; and the large size of the vessels involved, which do not constrict in response to the vasopressin. However, rebleeding is common. Initial control of upper gastrointestinal bleeding with embolization can be achieved in 84%; however, rebleeding occurs in 27%.
Esophageal Varices
Portal hypertension develops from an increase in resistance to portal venous outflow from both mechanical and vascular factors and is maintained by a systemic hyperdynamic circulation and peripheral vasodilation. A hepatic venous pressure gradient of at least 10 mm Hg is required for the development of esophageal varices, and a gradient of at least 12 mm Hg is generally required for the development of variceal bleeding. Gastroesophageal varices are present in approximately half of cirrhotic patients, and variceal hemorrhage occurs at a rate of 5% to 15% per year. Although survival has improved in recent years, variceal bleeding is associated with a mortality of at least 20% at 6 weeks. Variceal size, endoscopic stigmata, and advanced liver disease are predictors of variceal bleeding.
Prophylactic treatment of patients with varices without bleeding is recommended with nonselective β-adrenergic blockers, which decreases portal venous pressure. These agents should be used in patients with small varices with a higher risk of bleeding, specifically those with red wale signs or those with advanced liver disease. Bleeding prophylaxis is similarly recommended for patients with large varices with either β blockade or variceal band ligation.
Therapy for acute esophageal variceal bleeding includes vasoactive drug treatment (octreotide in the United States) for 2 to 5 days, endoscopic variceal band ligation, and prophylactic antibiotics. A percutaneously placed TIPS is used for the 10% to 20% of patients who fail to respond to the standard therapy. In those with advanced liver disease, TIPS may potentially have a survival advantage over standard therapy. Endoscopic variceal band ligation with or without β blockade is used for the prevention of rebleeding. Acute gastric variceal bleeding should be managed with pharmacologic therapy and antibiotics as well. Gastroesophageal and fundic varices can undergo banding acutely. Isolated gastric varices can be treated in the face of active bleeding with cyanoacrylate glue (not currently available in the United States). TIPS is used to prevent rebleeding from gastric varices. There are concerns with TIPS regarding shunt clotting, worsening hepatic encephalopathy, and deterioration of liver function.
Gastritis
Gastric mucosal bleeding attributable to stress, alcohol abuse, and use of nonsteroidal anti-inflammatory agents accounts for almost 30% of major gastrointestinal hemorrhage. The superficial erosions result from the breakdown of the mucosal barrier, allowing back diffusion of acid into the submucosa. Most patients with diffuse gastritis stop bleeding spontaneously. If they do not, transcatheter therapy should be attempted because surgery has a high mortality rate (21%) in this setting.
Diffuse hyperemia of the mucosa is seen angiographically in patients with hemorrhagic gastritis. If no extravasation of contrast medium is seen, intra-arterial injection of vasopressin should be performed. Embolization should be used in patients with active extravasation. These techniques are successful in approximately 80% of patients.
Mallory-Weiss Tear
Mallory Weiss syndrome is characterized by longitudinal mucosal lacerations in the distal esophagus and proximal stomach. Patients usually have a history of recent retching or vomiting and present with excruciating epigastric and left-sided chest pain radiating to the back. This condition accounts for approximately 5% of upper gastrointestinal tract bleeding. Diagnosis is made endoscopically.
Forty percent to 70% of patients with Mallory-Weiss syndrome require blood transfusion; however, 75% to 80% of patients are successfully treated with bed rest, sedation, and vigorous fluid and blood replacement. Endoscopic treatment, with the injection of epinephrine or ethanol followed by thermal coagulation, is successful in the majority of cases that fail conservative treatment. In rare cases in which endoscopy fails to staunch the hemorrhage, diagnostic and therapeutic angiography is indicated. After the bleeding vessel (usually a branch of the left gastric artery) is identified, a nonpermanent embolic agent such as Gelfoam pledgets should be used because this disorder is self-limited. Because the blood supply to the esophagus is complex, intra-arterial injection of vasopressin usually is not successful.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








