CHAPTER 21 Gastrointestinal interventions
Esophagus
Esophageal strictures
Etiology
Peptic disease is the most common cause of benign esophageal strictures1,2 (Box 21-1). Benign nonpeptic esophageal strictures are rare in routine clinical practice.
Box 21-1 Causes of BENIGN Esophageal Strictures
Adapted from Vetter S, Jakobs R, Weickert U. [Benign non-peptic esophageal stenosis: causes, treatment and outcome in routine clinical practice]. Dtsch Med Wochenschr 2010;135:1061.
Carcinoma of the esophagus is notoriously difficult to manage; approximately 60% of patients may only receive palliative treatment. Even among those cases that appear curable, many cases will recur and face a 5-year survival of approximately 20%.3 After surgical resection, almost 20% to 30% of patients have dysphagia secondary to recurrence of the disease or anastomotic strictures.4
Esophageal stricture dilation
Technique
Although most of these procedures can be performed under endoscopic guidance, fluoroscopy is instrumental in the setting of complex strictures because it aids not only in crossing the stricture but also in monitoring the actual dilatation. Several authors report reduced complication rates and improved therapeutic results when fluoroscopy is used.5,6 Very proximal esophageal lesions are difficult to cross with an endoscope and in these situations fluoroscopy becomes the only practical means of guidance.
Three general types of dilators are in use: mercury- or tungsten-filled bougies, over-the-wire polyvinyl dilators, and balloon catheters. The dilators and bougies range in size from 15 to 60 French (Fr) (5 to 20 mm), including Safe-Guide over-the-wire dilators, Weight-Right mercury-/tungsten-filled dilators/bougies, and Quantum TTC dilators. Esophageal dilatation balloons range in size from 8 to 18 mm in diameter and are available from various manufacturers (e.g., Boston Scientific, Cook Inc., ConMed, Medovations Inc.). These noncompliant balloons are similar to angioplasty balloons but are slightly less supple. The bougie-type dilators exert more radial and longitudinal force compared with the other two types. Except in instances in which longitudinal forces should be avoided, no clear advantage has been demonstrated among the dilator types.7
After moderate resistance is encountered during dilation therapy with Savary-type dilators, no greater than three consecutive dilators should be used in a single session.8 There are no objective data to guide balloon therapy; therefore, a conservative approach to dilation is usually undertaken to reduce the chances of perforation. Multiple dilation sessions frequently are required to achieve a luminal diameter of at least 12 mm.
Results
For benign strictures, balloon dilation is technically successful in 90% of cases. Clinical success is achieved in approximately 70% of patients at 2-year follow-up. The results are not as favorable for radiation strictures, and malignant strictures only show transient results.9,10 External compression of the esophagus rarely responds to dilation.
Although initial dilation typically results in symptomatic relief, recurrence of the stricture with return of symptoms is common. In the setting of peptic stricture, approximately 30% to 60% of patients require repeat dilations within a year.8 Several studies have attempted to identify prognostic factors associated with increased need for multiple dilations. Patients with peptic strictures who experienced weight loss or lacked symptoms of heartburn were more likely to require multiple sessions; however, the severity of the initial stenosis or the type and size of dilator used did not have an impact on recurrence.11
Malignant esophageal stricture dilation is both safe and effective.12,13 This intervention is performed either as an adjunct to endoscopic staging of esophageal tumors or as a temporary palliative treatment before surgery, placement of self-expanding stents, or laser photoablation.
Complications
Several early studies demonstrated a high complication rate associated with aggressive dilation of malignant esophageal strictures.14,15 The most serious complication following dilation is esophageal perforation. The reported rate is 0.1% to 0.4%.16,17 As expected, complex strictures have an increased risk for rupture. Contained esophageal perforations usually can be managed nonoperatively but occasionally may require surgical rescue.18–20
Mild bleeding is common following dilation and usually is self-limited. Severe bleeding occurs in 0.4% of patients.21 Inadvertent passage of the dilator into the trachea is a rare event. Unusual complications including discitis and epidural abscess have been described after both dilatation and stenting.21
Esophageal stricture stent placement
Patient selection
Laser therapy, radiation therapy, and placement of plastic stents have been used for palliation of aggressive esophageal strictures. Laser therapy offers effective palliation with a low rate of complications. However, it is not suitable for extrinsic compression, is difficult to perform if the narrowed region is long and tortuous, and requires repeated treatments with expensive equipment that is not readily available.22 Radiation therapy achieves palliation of dysphagia in less than 40% of patients and may take up to 2 months until dysphagia is relieved.23
Although rigid plastic stents are inexpensive and readily available, complications, (including perforation (8%), stent migration, tumor overgrowth, and bleeding) are not infrequent.24 Other treatments, such as steroid injection, hyperbaric therapy, and radial incision, have been used for tenacious strictures with sporadic success.
The indications for metallic stent placement in the esophagus include the following:
The indications for permanent esophageal stenting in patients with benign strictures have not been established. Some strictures, especially those caused by radiation or caustic ingestion, recur commonly after dilation and may benefit from stenting. Stents have been occasionally used in treatment of such resistant benign lesions.25 Plastic self-expanding stents are more commonly used in esophageal strictures because of their ease of placement and retrieval, and the limited local tissue reaction. More recently, biodegradable stents have been employed to manage benign esophageal stenosis.26
Recently, stents have been used for leaks and fistulas, with the potential benefits being healing without diversion or reconstruction and early return to an oral diet. Most commonly, covered stents are employed although bare stents can also be used in cases of small leaks or minute tears or fistulas.27,28
Technique
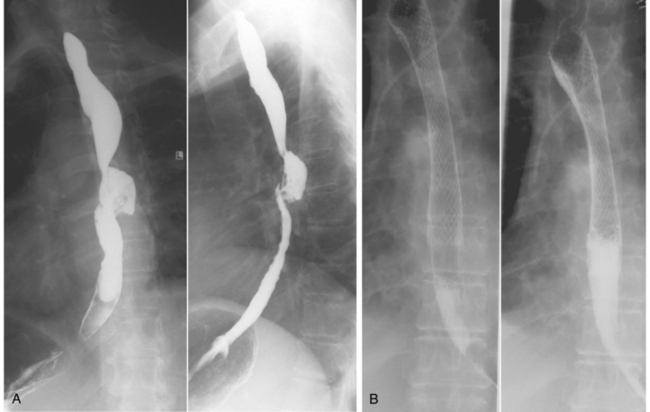
The stents approved by the U.S. Food and Drug Administration are the covered/uncovered Wallstent (Boston Scientific, Natick, Mass.), covered Gianturco-Rosch Z-stent (Cook Inc., Bloomington, Ind.), and covered/uncovered Ultraflex stents (Boston Scientific). The Wallstent has excellent radiopacity, has the highest radial force (more appropriate for extrinsic compression), and is easy to deploy (Fig. 21-1). However, it foreshortens during deployment, which must be taken into consideration when choosing the length of the stent. The Z-stent has good radiopacity, minimal shortening, and can accommodate an antireflux valve. The latter consists of a pressure sensitive “windsock type” valve created by extending the polyurethane covering over the edge at the esophageal end of the stent or a silicone membrane with a slit-type valve opening. The downside is that it lacks flexibility, requires a bulky sheath (up to 31 Fr), and is difficult to deploy. Greater stent shortening, poor radiopacity, and an unprotected delivery system make the Ultraflex stent deployment more difficult to insert than the Wallstent. The lower radial force and greater flexibility of the Ultraflex probably are responsible for less chest pain and better patient tolerance. It is the preferred stent for high esophageal lesions. Ultraflex has been shown to be equal in performance to Choostent.29,30 Newer stent designs include the SX-ELLA (Ella CS), which is fully covered to resist tissue in-growth and has an antimigration ring to withstand migration, and the controlled release Evolution stent (Cook Medical, Limerick, Ireland).31
Stents should never be placed across the superior esophageal sphincter (C5-C6 level) because of aspiration risk and patient discomfort.32 Crossing the gastroesophageal junction with the stent should also be avoided to prevent reflux.32 A transoral route is used commonly. Predilation is performed to facilitate rapid stent expansion and removal of the delivery system. If a fistula is present or suspected, dilation is avoided because of fear of enlarging the abnormal communication. After deployment, the stent should cover the lesion and have a safety margin of at least 2 cm of normal esophagus at each end. In an ideally positioned stent, the esophageal stricture produces a waist at the center of the stent.
After stent placement, patients need to modify their diets to prevent large boluses of food from becoming impacted within the stent. If a stent without a valve is used to cross the distal esophageal sphincter, the patient is placed on antireflux measures that include elevation of the head of the bed and pharmacologic acid suppression therapy.
Results
Esophageal stent placement is technically successful in 97% to 100% of cases.33 Overall procedure-related morbidity and mortality with self-expanding metallic stents are low.34–38 Dysphagia is relieved in 90% of patients who undergo esophageal stent placement.39 Successful closure of tracheoesophageal or bronchoesophageal fistulas can be achieved in 70% to 100% of patients.40 Treatment failure occurs more frequently when the esophagus is dilated, preventing stent-wall apposition, and when the fistula is in proximity to the superior esophageal sphincter. Overall, the reintervention rate is 25%.32
Complications
Adverse events related to esophageal stent placement are uncommon. Major complications include bleeding (up to 6%), aspiration pneumonia, tracheal compressions, perforations, and tracheoesophageal fistulas (up to 8%). Minor complications include chest pain/discomfort, tumor in-growth or overgrowth, stent migration (up to 35%), gastroesophageal reflux, hiccups, foreign body sensation, failure in stent expansion, granulation tissue formation, and food bolus obstruction (4% to 18%).41,42 Vocal cord paralysis and stent fracture have also been reported.43,44 The mortality rate associated with esophageal stent placement is approximately 0.5% to 2%.39
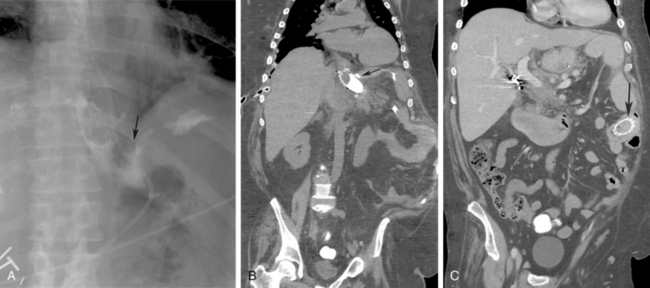
The most common complication seen with covered stents is migration, whereas the most common complication seen in uncovered stents is tumor in-growth (Fig. 21-2). Ileus and perforation have been reported with stent migration.45 To minimize migration, changes in stent design have been implemented that include covering inside the metal mesh (exoskeleton), uncovered segments at both ends (longer proximally), and larger proximal diameter (cone-shaped stent). A device with some of these modifications is the Flamingo stent (Boston Scientific).
Stomach
Enteral nutrition and decompression
Background
Malnutrition is a common problem affecting up to 40% of hospitalized patients, increasing their morbidity and mortality.46 Nutritional support can be given either enterally or parenterally. Parenteral nutrition is costly and has high risks of complications, including sepsis, hepatic dysfunction, metabolic disturbances, catheter-associated vein thrombosis, and intestinal mucosal atrophy.47 Several studies have shown that enteral feeding has lower complication rates when compared with its parenteral counterpart.48,49
Enteral feeding requires a functioning gut and can be provided in the short term via nasogastric or nasojejunal tubes. In the long term, however, these tubes have high complication rates, including mechanical problems, local erosions and aspiration pneumonia. Patients with chronic swallowing disorders or obstruction to swallowing, or those who are unable to ingest food because of head trauma or stroke, or those with anorexia resulting from underlying diseases such as cancer require more permanent access by placement of a gastrostomy, gastrojejunostomy, or jejunostomy.
Minimally invasive gastrostomy can be performed using endoscopic (percutaneous endoscopic gastrostomy [PEG]) or fluoroscopic (percutaneous gastrostomy [PG]) guidance. The advantage of PG over PEG is that endoscopy is avoided, which is a significant advantage in patients with an esophageal or pharyngeal obstruction. In addition, sedation requirements are less during PG. Wollman and colleagues published a comparison of surgical, endoscopic, and fluoroscopic techniques for gastrostomies that included a metaanalysis of the literature.50 Their conclusion was that PG is associated with a higher success rate than is PEG and with less morbidity than either PEG or surgery. More recently, Hoffer and colleagues performed a prospective, randomized comparison and concluded that percutaneous gastrojejunostomy had a higher success rate and fewer complications, whereas PEG took less time, cost less, and required less tube maintenance.51
Percutaneous gastrostomy (online video 21-1)
Patient selection
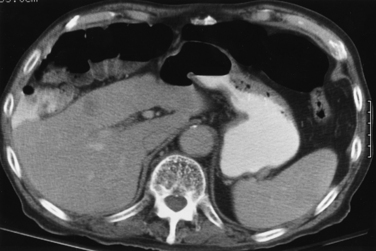
The major indication for PG as a means of nutritional support are listed in Box 21-2. PG also may be used for gastrointestinal decompression. This indication is more common in patients with carcinomatosis causing chronic intestinal obstruction. Less common indications include craniofacial abnormalities and impaired intestinal absorption, requiring slow infusions of alimental diet into the small bowel via a gastrojejunostomy or jejunostomy.52 Absolute contraindications to PG include uncorrectable coagulopathy and unsatisfactory anatomy (Box 21-3 and Fig. 21-3), such as an overlying colon or left lobe of liver or the entire gastric lumen located above the costal margin even after air insufflation.
The presence of a ventriculoperitoneal (VP) shunt was considered a contraindication, but this notion has been challenged. One study found a 9% VP shunt infection rate 1 month after PEG placement.53 Another study found that PEGs placed between 1 and 2 weeks after shunt placement caused no infections.54 Finally, Taylor and colleagues performed 16 shunts in 13 patients requiring gastrostomies (half before and half after PEG) and found a 50% shunt infection rate overall.55 They concluded that the development of infection was not related to the sequence or interval between shunt and gastrostomy placement and recommended avoiding simultaneous placement in the acute phase of a patient’s hospital admission. These are small series that include only PEG procedures, limiting our ability to reach conclusions or extrapolating these results toward the safety of PG in conjunction with VP shunt placement.
Placing PG or percutaneous gastrojejunostomy (PGJ) in patients with short life expectancies is debated in the medical literature. The mortality rate after PG is highly dependent on the patient’s underlying conditions (e.g., advanced age, malignancy, diabetes mellitus, and pulmonary disease).56,57 It is estimated that the cost of hospital visits for tube dislodgment or malfunction is almost $11 million per annum in the United States.58 Some argue that if no physiologic benefit is expected or if the improvement in physiologic status has no effect on quality of life (e.g., permanent vegetative state), then there is no obligation to offer or perform this intervention.59 The issue is controversial and depends on the philosophy of the institution, the physician in charge, the individual who inserts the tube, and the wishes of the patient and the family.
Device selection
There is a wide variety of gastrostomy and gastrojejunostomy tubes that differ in both size and retention mechanisms. No single gastrostomy catheter is appropriate for all patients (Fig. 21-4). The various types of gastrostomy options range from the simple pigtail configuration, such as the Deutsch gastrostomy (Cook Inc.), to balloon-retained devices such as the MIC tube (Kimberly Clark). “Button” gastrostomy tubes are popular for children. They lie flush with abdominal wall and have a very short external stem, making them much more cosmetically acceptable. However, with the increasing frequency of long-term gastrostomy placement in adults who are otherwise mobile, these are being used more often in adults as well.60
Technique
A fasting period of 8 hours is required before the procedure (Fig. 21-5). Any available cross-sectional imaging studies should be evaluated to determine suitability and best site for PG placement. Knowledge of any prior abdominal surgery is critical. Barium frequently is administered the day before for colonic opacification, and ultrasound is used to delineate the liver contour. A nasogastric tube is inserted and used for stomach insufflations during the procedure (Fig. 21-6). A small, 4-Fr angiographic catheter will suffice. Glucagon 1 mg intravenously will close the pylorus and help retain injected air in the stomach; however, it may increase the difficulty in crossing the pylorus when placing a gastrojejunostomy tube. Patients with prior gastric resection, upper abdomen interventions, esophageal obstruction, or a nondistensible stomach due to carcinomatosis may require computed tomography (CT) to assist in stomach identification and puncture (Fig. 21-7). Prophylactic antibiotics (e.g., cephazolin 1 g intravenously) are given by some interventionalists based on PEG studies.61
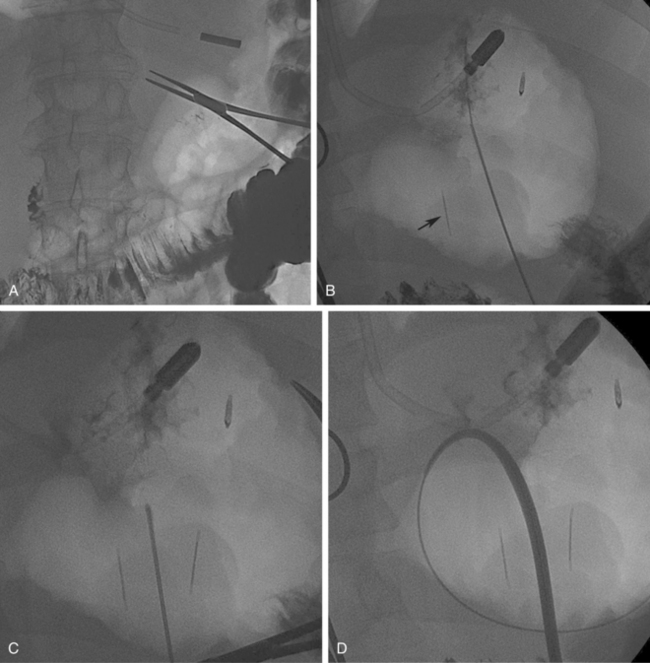
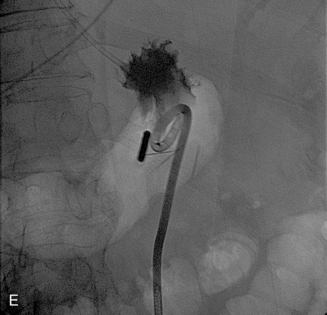
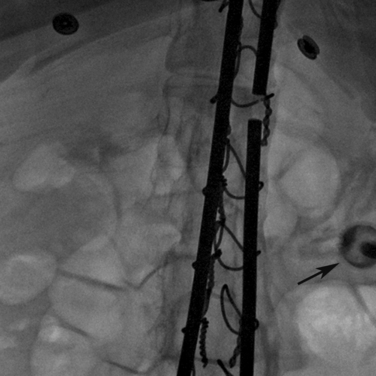
Figure 21-5 Steps in percutaneous gastrostomy tube placement. A, Initial localization. The hemostat marks the costal margin. Stomach is inflated with air via a preexisting nasogastric tube. The optimal access is through the greater curvature in the lower mid body of the stomach. B, Gastropexy using T-fasteners. Image shows first T-tack already in place (black arrow). A second anchor is being deployed through an 18-gauge needle. Intraluminal position is confirmed by contrast injection to outline the rugae as well as by lateral fluoroscopic imaging. C, Needle entry for actual tube placement into the stomach. Note the two T-fasteners already in position; some operators prefer to place three fasteners. Needle puncture directed toward the pylorus would simplify future conversion to gastrojejunostomy. Care is taken to be well below the costal margin and mindful of the expected location of the superior epigastric artery. D,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree