CHAPTER 16 Inferior vena cava
Inferior venacavography
Number 4- or 5-French (Fr) pigtail (or similarly shaped) catheters are used for venacavography with the side holes placed at the common iliac vein confluence. Sizing catheters with radiopaque markers assist in determining caval diameter in patients undergoing IVC filter placement. When iodinated contrast must be avoided because of renal insufficiency or history of severe contrast allergy, CO2 is used.1 A typical injection rate with iodinated material is 15 mL/sec for 2 seconds. With CO2 venography, rapid manual injection of 40 to 60 mL is necessary for adequate opacification. CO2 venography is prohibited in patients with a documented right-to-left circulatory shunt.
Anatomy
Development
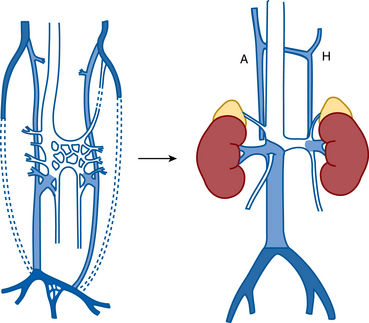
The posterior cardinal veins drain the caudal aspect of the fetus during the first weeks of development.2,3 By the seventh week of gestation, these vessels start to involute, and the subcardinal and supracardinal venous channels emerge (Fig. 16-1). In the middle of the abdomen, the paired systems fuse into a vascular sinus, the subcardinal-supracardinal anastomosis. The normally positioned right-sided IVC and the azygous and hemiazygous venous circulations arise from the subcardinal and supracardinal vessels.
Normal anatomy (online case 11)
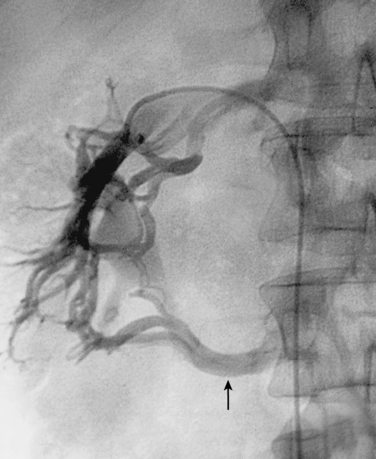
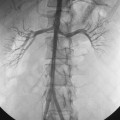
The IVC begins at the confluence of the common iliac veins, which corresponds to the L4-L5 level2,4 (see Fig. 16-1 and Fig. 16-2). The left common iliac vein and iliocaval junction pass between the right common iliac artery and the spine at this site. The IVC ascends in the retroperitoneum to the right of the aorta. In the midabdomen, the IVC courses behind the head of the pancreas, the superior portion of the duodenum, and the portal vein. The vessel then runs in a groove on the posterior surface of the liver. As it traverses the diaphragm, it is enveloped by the pericardium and then enters the inferoposterior aspect of the right atrium (RA). The eustachian valve is located on the right lateral side of the IVC at its junction with the RA. Otherwise, the IVC has no valves. The ascending lumbar veins originate from the common iliac veins and run alongside the vertebral bodies (Fig. 16-3). Above the L2 level, they drain into the azygous system (see later discussion). Four pairs of lumbar veins connect the IVC with the bilateral ascending lumbar channels (Fig. 16-4).
The renal veins empty into the IVC about the L1-L2 level (see Figs. 16-2 and 16-4). Renal vein anomalies (e.g., multiple veins, circumaortic or retroaortic left renal vein) are seen in up to 30% of individuals5 (Fig. 16-5; see also Figs. 10-8 and 10-9). The right gonadal vein enters the IVC just below the right renal vein, and the left gonadal vein enters the undersurface of the left renal vein lateral to the vertebral column (see Fig. 16-4). The right adrenal vein drains into the right posterior wall of the suprarenal IVC in a horizontal plane at about the T12 level (see Figs. 13-8 and 13-9). In contradistinction, the left adrenal vein joins the left renal vein in a vertical plane as a common trunk with the left inferior phrenic vein (see Fig. 16-4; see also Fig. 10-3).
The right, middle, and left hepatic veins converge at the upper IVC just below the diaphragm (see Fig. 12-4). In many individuals, more caudally positioned accessory hepatic veins drain portions of the right or caudate lobes of the liver. In about 3% of the population, blood from the right lobe of the liver empties primarily through a large inferior right hepatic vein that is situated on the IVC well below the other hepatic veins6 (see Fig. 16-4).
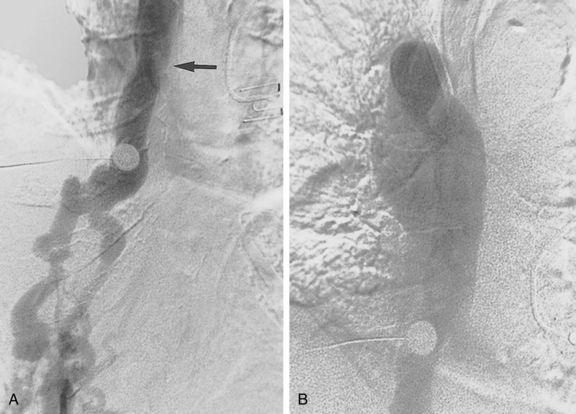
The azygous venous system drains the posterior chest wall, esophagus, mediastinum, and pericardium. It also serves as an important collateral circulation for the IVC and tributaries when they become obstructed (Fig. 16-6). The azygous vein originates at the L1-L2 level as an indirect continuation of the right ascending lumbar veins. It then ascends through the abdomen and chest on the right side of the spine. At about the T4-T5 level, it arches anteriorly to join the back wall of the superior vena cava (SVC). The hemiazygous vein also begins at the L1-L2 level and runs on the left side of the spine. It crosses the midline to join the azygous vein at about the eighth thoracic vertebra. The accessory hemiazygous vein, which arises from the left brachiocephalic vein, joins the SVC just above the hemiazygous connection.
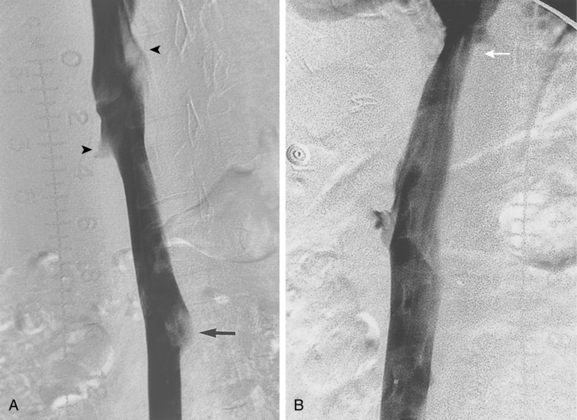
The mean transverse diameter of the infrarenal IVC in adults is about 19 mm.5,7 Caval diameter usually decreases during inspiration or a Valsalva maneuver, and it usually increases during expiration.8 On cross-sectional images, the IVC often appears elliptical rather than round in the transverse plane. Mild or moderate narrowing of the intrahepatic IVC is common (see Fig. 16-2B). Inflow defects at cavography caused by unopacified blood from the renal, hepatic, and contralateral iliac veins should not be confused with clot, which does not change appearance on sequential images (see Fig. 1-39). On the other hand, retrograde filling of the IVC (on contrast-enhanced computed tomography (CT)) or prominent filling of primary IVC branches (at cavography) is a sign of elevated right heart pressures from a variety of causes.9
Variant anatomy (online cases 49 and 71)
The major IVC anomalies can be explained by errors in the normal fetal evolution of the major veins of the abdomen and pelvis3,4,9–11 (see earlier and Fig. 16-1).
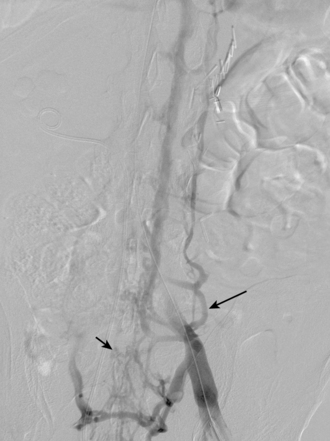
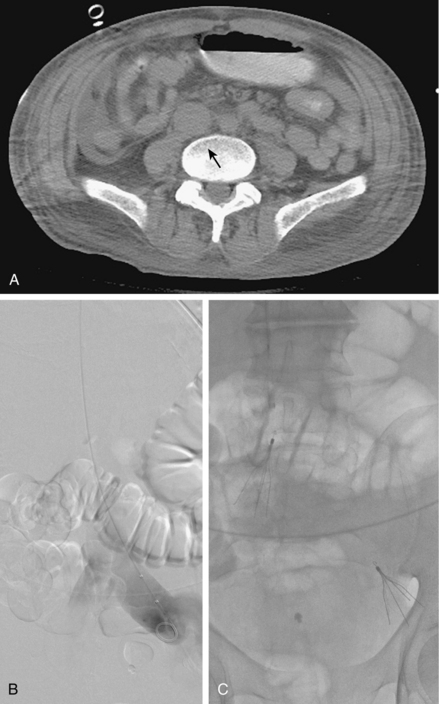
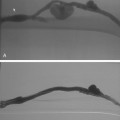
Duplication of the IVC results from persistence of the inferior portion of the left supracardinal vein. The quoted prevalence of this anomaly varies from 0.2% to 3%.4,12 Each iliac vein drains into its corresponding vena cava (Fig. 16-7). The left IVC usually joins the right IVC at the level of the left renal vein. The right and left common iliac veins sometimes communicate in the pelvis. At venacavography performed from the right femoral vein, IVC duplication may be suspected by the absence of an inflow defect from the left iliac vein and by the small caliber of the infrarenal IVC. However, it can go undetected on routine cavograms; if the initial study is suspicious, catheterization of the left iliac vein is advisable.
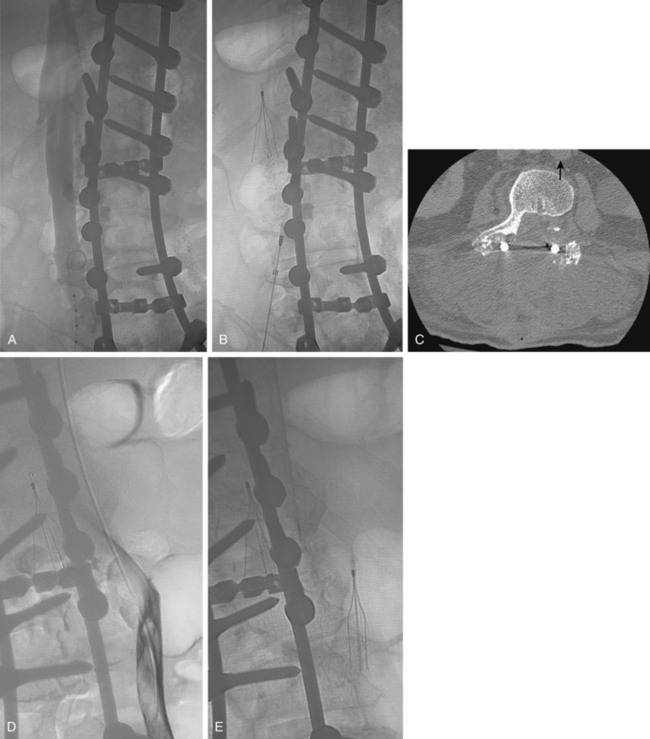
Figure 16-7 Duplicated inferior vena cava (IVC). A, Cavography prior to inferior vena cava filter placement did not suggest variant anatomy, so a single filter was placed (B). By serendipity, a colleague later noted IVC duplication at the edge of an image on a lumbar spine computed tomography study (arrow)(C). D, Left iliac venography confirms duplication and presence of large acute thrombus. E, From a jugular approach, a second Guenther tulip filter was placed in the duplicated moiety above the thrombus.
Left (transposed) IVC results from persistence of the inferior portion of the left supracardinal vein with involution of the inferior portion of the right supracardinal vein. In autopsy series, left IVC is found in 0.2% to 0.5% of cases; a similar frequency (0.2%) has been reported in imaging studies.4,12 Usually, the left IVC joins the orthotopic IVC at or just inferior to the left renal vein (Fig. 16-8). Occasionally, it may join the azygous or hemiazygous system.13 Very rarely, it connects with a left SVC, which ultimately drains into the coronary sinus.14
Interruption of the IVC with azygous or hemiazygous continuation occurs when the right subcardinal vein fails to join the intrahepatic venous complex. IVC interruption is found in 0.6% of autopsy specimens but less often in imaging series.4,10,15 In this situation, blood flows from the lower IVC into the azygous or hemiazygous vein and then into the heart (Fig. 16-9). The hepatic veins drain directly into the RA.2 The anomaly often is associated with congenital heart disease and with abnormalities of the spleen (i.e., asplenia or polysplenia), cardiac position, and abdominal situs.16,17
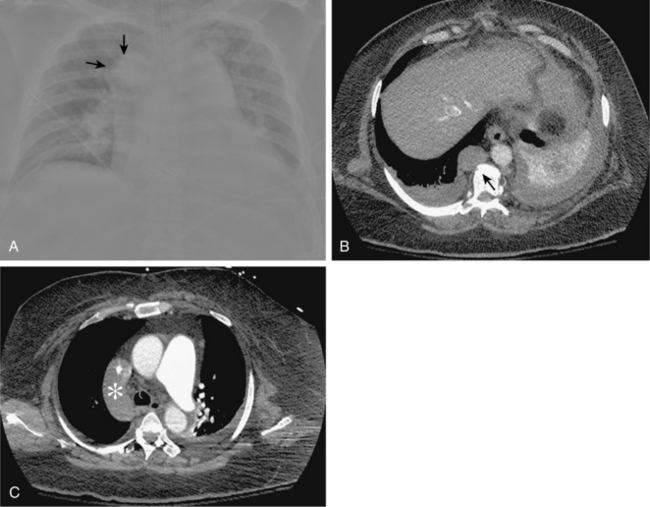
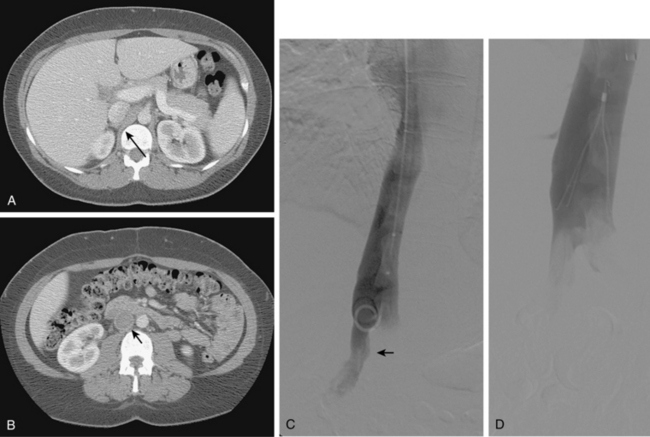
Figure 16-9 Azygous continuation of the inferior vena cava (IVC). A, Chest radiograph shows large round density lateral to the right main stem bronchus (arrows) at the site of the azygous arch. B, Contrast-enhanced computed tomography scan shows a massively dilated azygous vein (arrow), which continues up the chest and drains directly into the superior vena cava (C,asterisk).
Other exceedingly rare caval variants include double IVC with retroaortic right renal vein and hemiazygous continuation of the IVC, double IVC with retroaortic left renal vein and azygous continuation of the IVC, and absent infrarenal IVC with preserved suprarenal segment.4
Collateral circulation
Several alternate pathways allow blood to return to the heart when there is severe stenosis or occlusion of the IVC.18 The most common collateral route is the ascending lumbar venous plexus (see Fig. 16-3). These vessels also communicate freely with an extensive vertebral venous network. Blood flows cephalad through these veins into the azygous or hemiazygous systems and then into the SVC (see Fig. 16-6).
Major disorders
Inferior vena cava filter placement for venous thromboembolic disease (online cases 23, 49, and 71 and video 16-1)
A filtering device is inserted into the IVC for only one reason: to prevent clinically relevant pulmonary embolism (PE).19,20 However, IVC filters are not appropriate substitutes for standard medical therapy of venous thromboembolic disease (VTE) in patients who are reasonable candidates for anticoagulation (see later discussion and Chapter 15). In certain clinical situations, virtually every knowledgeable physician would support filter placement. Still, a substantial number of experts remain skeptical about their touted benefits (prevention of significant PE and associated reduction in mortality) and concerned about the associated risks (namely, the possible predisposition to deep vein thrombosis (DVT) and post-thrombotic syndrome).21,22
Patient selection
Box 16-1 outlines the indications for IVC filter insertion.23–28 In the setting of documented VTE (“proximal” lower extremity DVT or PE), the widely accepted criteria are (1) a preexisting contraindication to anticoagulants (see Box 3-7), (2) a suboptimal response to therapeutic anticoagulation, and (3) a complication from anticoagulant medications. Relative contraindications include severe and uncorrectable coagulopathy, absence of a suitable access route to the IVC, and chronic thrombosis of the vena cava. Although some interventionalists are reluctant to place a filter in a septic patient, the risk of seeding a filter is probably more imagined than real.23,29
Box 16-1 Indications for Inferior Vena Cava Filter Placement
Placement of an IVC filter as an entirely prophylactic maneuver in patients at risk for VTE (particularly before major surgery or after major trauma) is extremely controversial. Certainly, DVT causes substantial morbidity and mortality in trauma populations; one large study cited a 27% overall incidence of DVT despite adequate prophylactic anticoagulation.30 Both sides of the debate advance compelling arguments—substantial reductions in mortality from prophylactic insertion on the one hand, nonsignificant benefits and potential for exacerbating DVT on the other.31–34 Notably, the most recent policy guidelines on this subject from the American College of Chest Physicians advise against prophylactic placement even when the risk for VTE is deemed high.35
In a small subset of patients, both permanent IVC filter insertion and lifelong anticoagulation are deemed necessary to adequately reduce the lifetime risk for PE (e.g., before surgical thromboendarterectomy for chronic thromboembolic pulmonary hypertension, see Chapter 14). The frequently cited PREPIC study (see later discussion) has led many physicians to infer that filter placement should generally be avoided unless long-term anticoagulation will be instituted (when safe) for as long as the filter remains in place36; however, there are persuasive arguments that refute this widely held view.37
The practice of deploying a temporary filter before lower extremity venous thrombolysis varies considerably.38,39 One clinical study detected newly trapped clots during thrombolytic therapy in more than 50% of cases; another study failed to identify any such occurrences. Most interventionalists do not place filters routinely during this procedure, but instead reserve the device for high-risk situations (e.g., free-floating caval or iliac vein clot).
In rare circumstances, a suprarenal filter must be placed above the renal vein inflow40 (Box 16-2). Finally, SVC filter placement is discussed in Chapter 17.
It is noteworthy that the anticipated growth in IVC filter placement procedures following the introduction of retrievable devices has been partly offset by concerns about the added risk for lower extremity DVT and consequent post-thrombotic syndrome.41,42 The decision to implant a filter, even as a temporary measure, should be made carefully by the operator and patient. The ever-expanding indications for IVC filter insertion do not give license to referring physicians to request nor interventionalists to offer or agree to placement without solid evidence for the individual patient benefit.
Staging
Inferior vena cava diameter and length
About 1% to 2% of the general population has a megacava, whereby the transverse diameter exceeds 28 to 29 mm.43,44 In this situation, only the Bird’s nest filter (which expands to 40 mm) is guaranteed to remain in a stable position after release45 (Fig. 16-10). Alternatively, standard filters (permanent or retrievable) can be inserted into each common iliac vein (Fig. 16-11). The Bird’s nest filter system has a longer footprint than other devices (7 cm or greater); as such, the proposed infrarenal landing zone should be measured in advance.
Location and number of renal veins
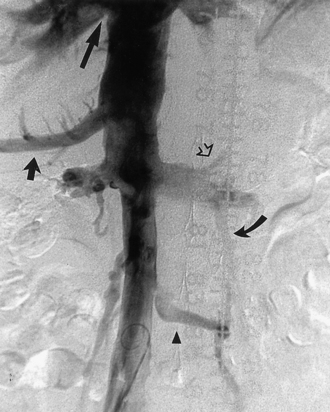
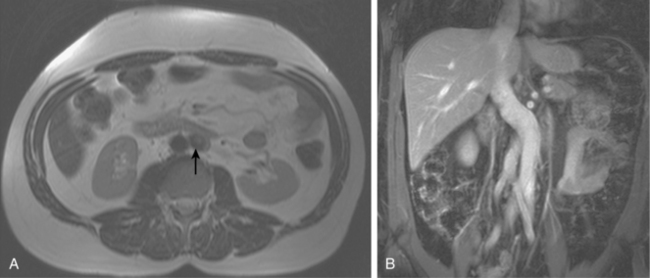
By convention, IVC filters are deployed with the most superior point immediately below the level of the renal veins. If the filter clots spontaneously (very unlikely) or becomes filled with emboli (much more likely), brisk blood flow from the renal veins will facilitate endogenous lysis of any clot at the top of the filter. Additionally, the lack of caval dead space above an occluded filter should minimize the likelihood of bilateral renal vein thrombosis. The position of the renal veins is identified by their inflow defects on the venacavogram (see Fig. 16-2). However, accessory renal veins are present in up to 25% to 30% of individuals (more frequently on the right than the left). These vessels are not always detected by venacavography alone. Appropriately performed CT or magnetic resonance imaging (MRI) may be helpful. If not, selective catheterization often is necessary to identify them5 (Fig. 16-12). Accessory renal veins must be distinguished from lumbar veins or the right gonadal vein. In patients with multiple renal veins (circumaortic or otherwise), the filter is deployed with the apex below the inferior-most vein. This approach prevents the more superiorly located vessel from acting as a conduit for emboli in transit to the lung in case the more inferior portion of the IVC becomes occluded (Fig. 16-13).
Inferior vena cava anomalies
Variants in IVC anatomy are sought (see earlier discussion). A duplicated IVC requires placement of filters in each moiety or suprarenal insertion of a single device (see Fig. 16-7).
Intrinsic disease
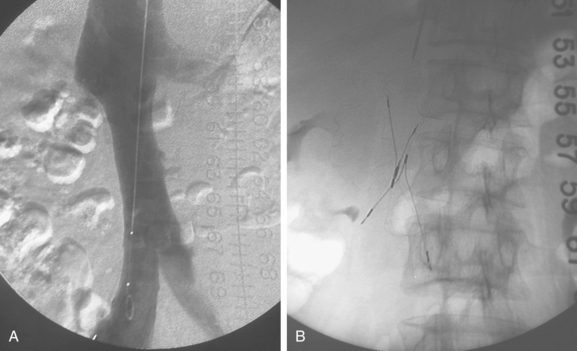
Filter location is influenced by the presence of intraluminal clot, chronic mural disease, or extrinsic compression. If caval thrombus is found, the filter is deposited just above the clot (when feasible) through the IJ vein (Fig. 16-14). Extension of clot into the IVC from tributaries may be a sign of occult malignancy (e.g., right gonadal vein thrombosis in ovarian neoplasms). Chronic total occlusion of the IVC possibly obviates the need for filter placement, although clots still can pass through enlarged collaterals. If suprarenal filter placement is required, it is critical to identify an appropriate landing site and to be certain that the nondiseased caval segment is long enough to accommodate the filter without extension into the RA (see Fig. 16-14).
Device selection
Before intraluminal IVC filtration devices were developed, operative caval interruption was achieved by ligation, stapling, plication, or clipping. However, subsequent IVC thrombosis was all too frequent.46,47 The Greenfield filter was the first widely successful intraluminal device, placed initially by open surgery and beginning in 1984 by percutaneous means.48,49
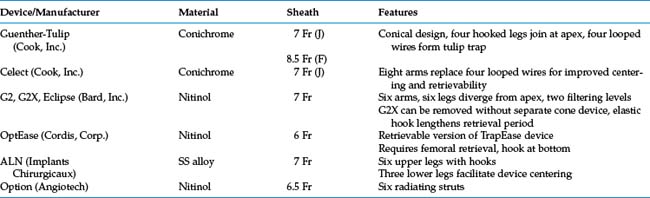
At present, a variety of permanent IVC filters are commercially available24 (Figs. 16-15 and 16-16 and Table 16-1). The critical properties of filters are clot-trapping efficiency, IVC and access vein occlusion rates, tendency for migration or embolization, mechanical integrity, and ease of placement. Despite decades of experimental and clinical studies comparing their relative merits (clot trapping ability vs. caval thrombosis rate), none of the currently used devices holds an unequivocal advantage.20,24,50–55 Certain filters seem to possess superior clot-trapping ability compared with the others.55–58 This attribute may be associated with higher rates of caval thrombosis, but it may make these devices more suitable for patients who cannot tolerate even small pulmonary emboli.
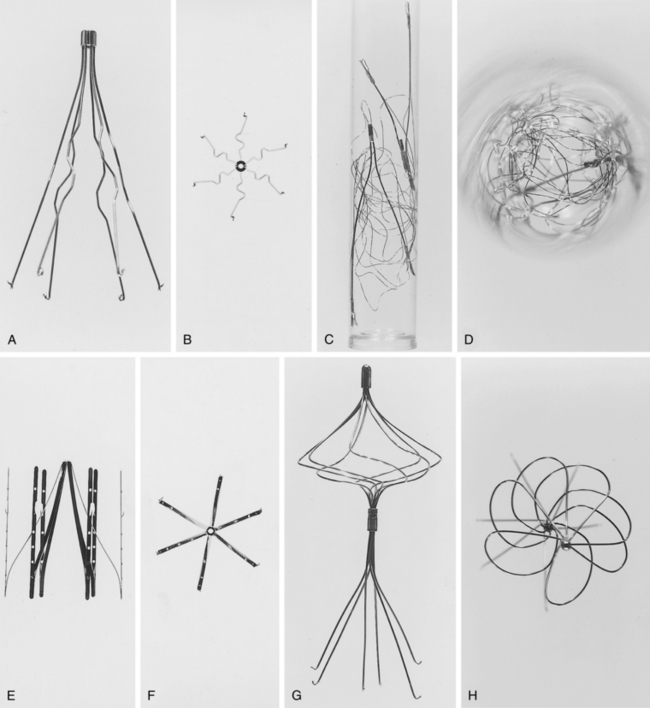
Figure 16-15 Frontal and aerial views of inferior vena cava filter designs. A and B, Stainless-steel Greenfield filter (Boston Scientific, Natick, Mass.). C and D, Bird’s nest filter (Cook Inc., Bloomington, Ind.). E and F, Vena-Tech LGM filter (B. Braun, Evanston, Ill.). G and H, Simon nitinol filter (Bard, Inc., Tempe, Ariz.).
Table 16-1 Permanent Inferior Vena Cava Filters
| Device/Manufacturer | Material | Sheath |
|---|---|---|
| Bird’s nest (Cook, Inc.) | Stainless steel | 12 Fr |
| Greenfield (Boston Scientific) | Stainless steel | 12 Fr |
| Simon nitinol (Bard, Inc.) | Nitinol | 7 Fr |
| TrapEase (Cordis Corp.) | Nitinol | 6 Fr |
| Vena-Tech LP (B. Braun) | Phynox | 7 Fr |
The more recently developed products that permit discontinuation of IVC filtration are termed optional filters, which fall into two categories.59Convertible IVC filters can be manipulated in situ to abolish their filtering capacity; none is currently commercially available in the United States. Retrievable IVC filters have the potential for complete removal. However, they are primarily approved by American and European regulatory agencies for permanent placement.
Table 16-2 lists and Fig. 16-17 shows currently available optional IVC filter designs.60,61 Again, none of these retrievable devices has emerged as clearly superior to the others. However, before selecting a particular filter, operators are cautioned to review the most recent data regarding postplacement filter migration and long-term integrity. Early versions of some optional filters had serious design flaws that required their removal from the market.
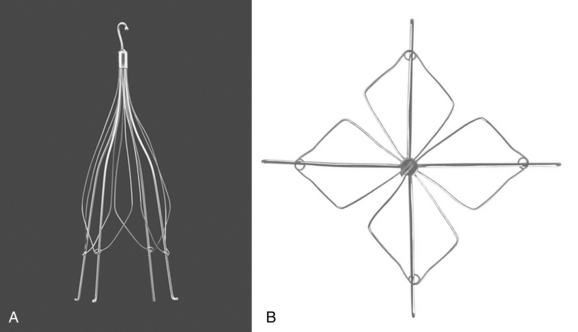
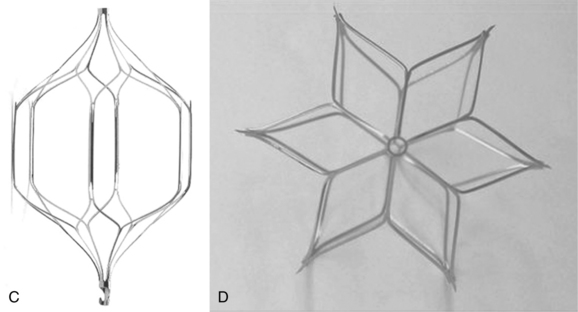
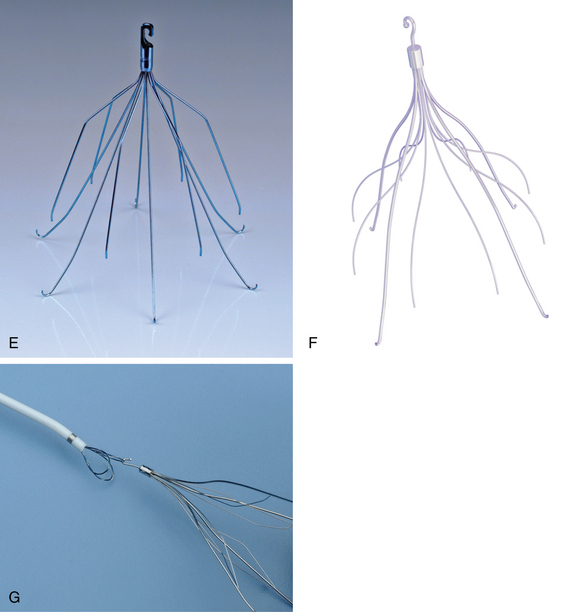
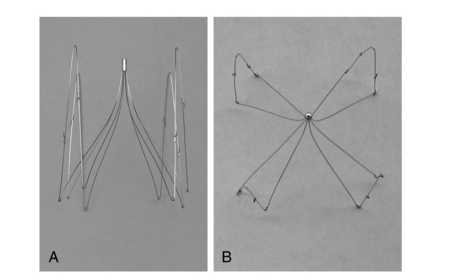
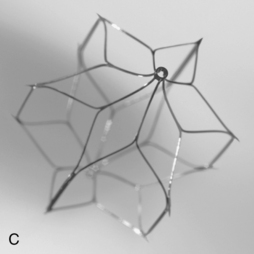
Figure 16-17 Retrievable inferior vena cava filters. A and B, Guenther-Tulip filter (Courtesy of Cook, Inc.). C and D, OptEase filter (Copyright Cordis Corp., 2011). E, Eclipse filter (Courtesy of Bard, Inc.). F, Celect filter (Courtesy of Cook Medical, Inc.). G, Guenther tulip filter with tri-lobed snare around retrieval hook (Courtesy of Cook Medical, Inc).
Box 16-3 outlines the typical scenarios that favor use of a retrievable filter.62–64 For the most part, this group includes patients with a limited and fairly predictable period of increased risk for VTE. When risks factors for VTE resolve or when anticoagulation can be instituted safely, the filter may be removed. Certainly, a young, previously healthy individual who suffers major trauma and VTE is an ideal candidate for a retrievable device.64,65 In fact, it might seem that having the option of filter removal is ideal for all patients. However, the evaluative period of optional filters is short compared with the decades-long experience with permanent devices. Thus, at present, if filter removal seems unlikely, a permanent device should be strongly considered.66
Insertion technique
IVC filters usually are deployed from the right IJ vein or a CFV. The IJ approach has several advantages (Box 16-4). In the unusual circumstance in which both femoral and IJ veins are occluded or unavailable, an antecubital, upper arm, or external jugular vein route can be used.67Box 16-2 outlines indications for suprarenal IVC filter placement.24,68–70 Suprarenal insertion is recommended in pregnant women to avoid compression of the device by the gravid uterus and to prevent PE if ovarian vein thrombosis develops.
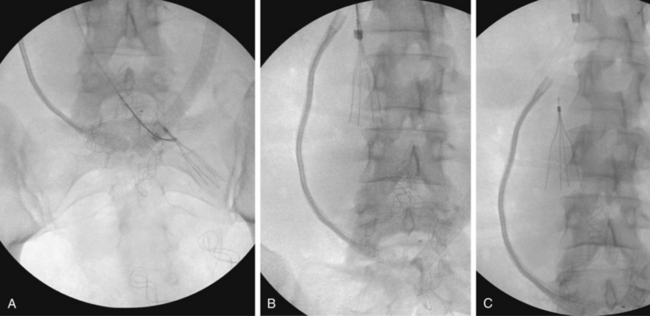
Bedside placement of IVC filters with intravascular ultrasound guidance has become quite popular in some centers. This approach may be especially advantageous in unstable patients confined to the intensive care unit.71–73 On occasion, however, the lack of fluoroscopic confirmation leads to malplacement of the device (e.g., in an iliac vein; Fig. 16-18).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree