CHAPTER 19 Hemodialysis access
Access construction
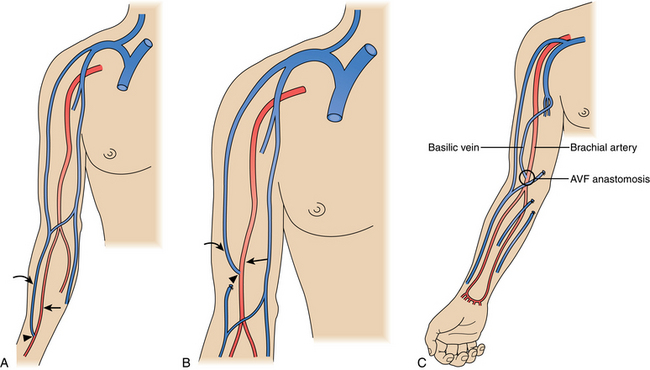
The ideal hemodialysis access is an endogenous fistula created by surgical anastomosis of an artery and vein. The updated National Kidney Foundation Kidney Dialysis Outcomes Quality Initiative (NKF-KDOQI) and clinical practice guidelines have set a goal of primary arteriovenous fistula construction in at least 65% of all new patients with kidney failure, with ultimately 65% of hemodialysis patients having a native fistula.1 Arteriovenous (AV) accesses are placed as far distally in the upper extremity as possible to preserve proximal sites for future access. Ideal access is within the upper extremity and preferentially within the nondominant arm if opportunities exist.
Although an autologous arteriovenous fistula (AVF) is the ideal hemodialysis access, it may be difficult or impossible to create successfully in a significant number of patients. Failures are particularly common in older and diabetic patients. Still, with careful evaluation even these high-risk patients may be suitable for a fistula.3 The reported failure rates range from 10% to 65%, but the usual figure in modern practice is about 15% to 25%.3–8
Because a fistula requires 3 to 4 months to mature, it should be created well in advance of the anticipated need for dialysis.1,9 The veins in the arm must not have been damaged by previous venipunctures. Fistulas are ready for use when the blood flow is greater than 600 mL/min, vein diameter is at least 0.6 cm, depth under skin is less than 0.6 cm, and there are discernible vein margins under the skin.1 In one postoperative study, fistulas with a diameter of at least 4 mm and a blood flow greater than 500 mL/min had a 95% likelihood of success for dialysis.10 If an endogenous fistula can be constructed and matures properly, it should have excellent long-term patency.
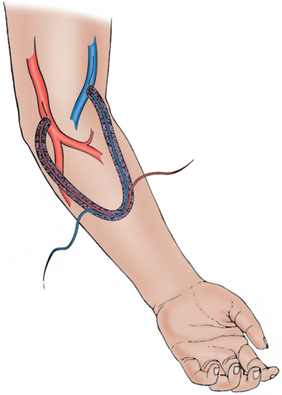
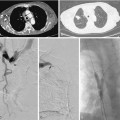
A synthetic graft is the alternative to an endogenous fistula. These grafts are commonly made of polytetrafluoroethylene (PTFE). They may be placed in the forearm in a straight configuration from the radial artery to a brachial vein, but a looped configuration from the brachial artery to a brachial vein (Fig. 19-3) usually is preferred.1 Grafts also may be constructed in the upper arm, usually in a looped configuration from the brachial or axillary artery to a high arm vein. If all possible sites in the arm and chest are exhausted, a loop graft can be placed in the thigh from the common femoral artery to the common femoral vein.
PTFE grafts may be used much earlier than native fistulas, usually within 14 days of placement.1 However, they do not have the longevity that can be achieved with an endogenous fistula. Primary patency rate at 1 year is reported to be approximately 40%.4,11 Secondary patency rate ranges from 60% to 90%.1,12 The most common reason for failure is development of a stenosis in the outflow vein, leading to thrombosis of the graft.1
Major disorders
Immature or failing dialysis access (online cases 60 and 72)
Etiology
Some dialysis AVFs fail to become usable for dialysis. Causes of an immature fistula are outlined in Box 19-1. Ultrasound may be very helpful in evaluating the dysfunctional access and identifying the source of the problem. On physical examination, it may be possible to determine the etiology based on the pulse or thrill within or caliber of the outflow vein(s). Multiple small veins with a palpable thrill may be present instead of a single dominant vein. In other cases, the fistula may have developed but may have decreased flow and appear flat, suggesting a problem in the juxtaanastomotic region. If the vein is dilated in one area but then becomes flat further up the arm, an outflow problem is indicated.
Mature but failing arteriovenous fistulas may have multiple, tandem stenoses at various points in the access circuit. The clinical consequences include large collateral veins, arm swelling, and inefficient dialysis from high venous pressure and low flow (Fig. 19-4). Synthetic dialysis grafts most often fail because of a venous anastomotic stenosis. In both types of access, veins that are subjected to the high flows, high pressures, vibratory and stretching effects, compliance mismatch, and turbulence are prone to develop stenoses from neointimal hyperplasia.13–15 Regardless of the cause, the venous stenoses progressively worsen until blood flow is markedly reduced and thrombosis occurs. These venous lesions often recur (again and again) at the same site.
Surveillance and imaging
The NKF-KDOQI guidelines recommend an organized monitoring program to identify access in jeopardy of thrombosis by regular assessment of several clinical and functional dialysis parameters.1 Proper clinical evaluation should be performed at least monthly and consists of inspection, physical examination, and auscultation of the fistula or graft. Although there continues to be controversy regarding the benefit of vascular access surveillance, surveillance can be performed in a number of direct and indirect methods. Direct monitoring may include intra-access flow, static venous dialysis pressure, and dynamic venous pressure.1,9,16–19 Other studies that are used to detect arteriovenous graft stenosis include measurement of access recirculation, decreases in the measured amount of hemodialysis delivered, or elevated negative arterial pre-pump pressures that hinder blood flow.1,20–22 Normal flow in a graft should be in excess of 800 to 1000 mL/min, and if flows decrease to less than 400 to 600 mL/min, risk of failure is increased.1,23,24 Clinical indicators include changes in the physical characteristics of the graft thrill, difficulty with needle placement, prolonged bleeding after needle removal, swelling of the arm, or, ultimately, clotting of the graft.1,25–27
Persistent abnormalities in any of these parameters suggest graft dysfunction and should prompt venographic evaluation of the graft. Venography can be performed easily after dialysis through the indwelling needles or can be performed at a separate session, usually before dialysis. If a stenosis is discovered at the time of the venogram, angioplasty can be performed immediately. Early correction of venous stenoses reduces thrombosis rates. In a number of studies, a 50% to 90% decrease in the thrombosis rate can occur when monitoring is instituted28,29 and prolongs access viability.1,9,17,30,31
Surveillance for AVFs depends on many of the same methods as those used for grafts. Physical examination is very helpful and may be even more informative than with grafts.32 Ultrasound can be used both preoperatively to increase the rate of successful fistula placements33 and to evaluate fistula maturation.34 The other measures of graft function (recirculation, increased static venous pressures, decreased flow rates) are also evidence of fistula malfunction, but the thresholds for abnormality are not well established. As a result of the low resistance and presence of the collateral veins, a venous stenosis causes a reduction in blood flow in a fistula without the corresponding increase in access pressure.35
Endovascular therapy
Patient selection
The results of both percutaneous and surgical repair of failing dialysis access are disappointing. Several studies have reported comparable primary patency rates at 6 months (less than 25%) and 12 months.29,30 The NKF-KDOQI advises that each dialysis center determine which procedure is best for an individual patient based on local expertise. Surgical revision is held to a higher standard than percutaneous transluminal angioplasty, because surgical revision usually extends the access further up the extremity by the use of a jump graft.1
Although angioplasty is plagued with the same problems with recurrent stenosis as surgery, it does have some advantages. Patients accept percutaneous balloon angioplasty better than they do a surgical procedure. The angioplasty procedure avoids using a site further up the venous outflow, leaving it available for subsequent revision when it becomes necessary. The graft is immediately available for dialysis, avoiding the need for temporary dialysis catheters. Redilations allow easy and safe graft salvage for months or years.25 New data also demonstrate promising results with stent grafts at graft-related stenoses.36 Percutaneous revision of venous anastomotic stenoses within a prosthetic hemodialysis graft with the use of a stent graft appears to provide superior long-term patency than standard balloon angioplasty.
Technique
Dialysis graft/fistula venography should include a complete evaluation of the circuit from the inflow artery to the superior vena cava. Access is usually done by palpation in the direction of suspected disease. Evaluation of fistulas can be more complicated. A variety of techniques can be used to help with a difficult access. Real-time ultrasound guidance is extremely helpful. A tourniquet can be used to impede outflow and increase the size of the target veins. If direct fistula access fails, the brachial artery is punctured with a micropuncture needle central to the anastomosis. The 3-French (Fr) inner dilator of the micropuncture set allows diagnostic angiography and minimizes the risk for complications.37–41 Direct arterial access also allows evaluation of the palmar arch and antegrade or retrograde filling of the distal segment of the artery feeding the fistula.37 After the diagnostic study has been performed, the fistula can be cannulated by a retrograde approach.37
Venous stenoses in fistulas are seen with both immature and mature accesses. Turmel-Rodrigues and colleagues42 think these venous stenoses must be dilated to at least 5 mm to obtain adequate flows.42 However, in a poorly developed fistula, it may be important to perform serial dilations, starting with undersized balloons (3 to 4 mm) to avoid rupturing a small, fragile vein. If the vein is not well developed, dilation with a small balloon during one procedure and a repeat evaluation in 1 to 3 weeks with dilation using a larger balloon may allow for development of the venous outflow and maturation of the fistula (see discussion below). One of the more common sites of stenosis in a brachiocephalic fistula involves the cephalic arch region with a 39% prevalence of stenosis.43,44 This region is less problematic with a radiocephalic fistula with an overall prevalence of 2%.43 Traditional angioplasty results within this region have been disappointing, with primary patency at 3, 6, and 12 months at 96%, 83%, and 75%, respectively.44 These outcomes have been in part due to the resistant nature of the stenosis, early restenosis, high vein rupture rates, and other anatomic and hemodynamic considerations.45
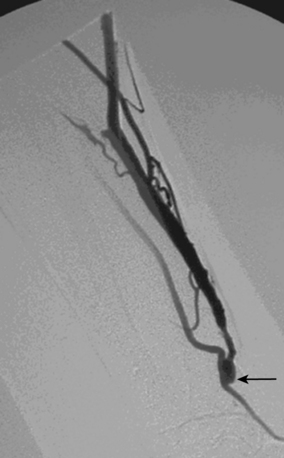
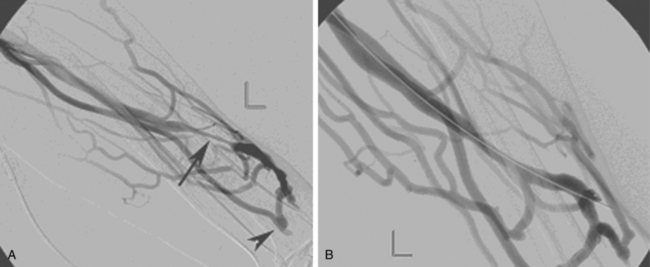
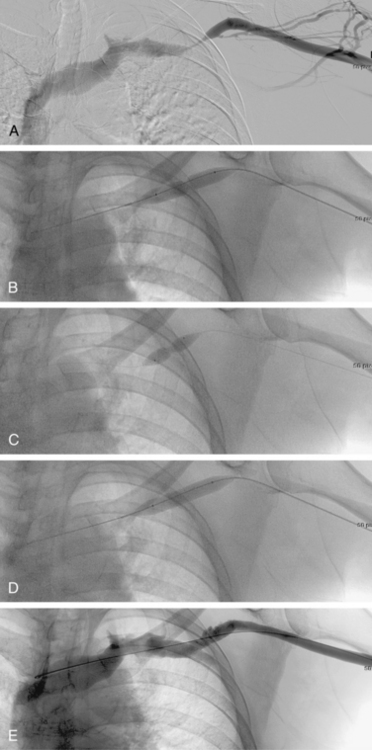
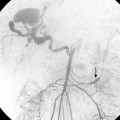
As many as 30% of native fistulas never mature to enable cannulation and allow for successful hemodialysis.46–49 If a fistula fails to mature over the first 1 to 3 months, early fistulography can enable identification of an underlying stenosis for endovascular treatment (Fig. 19-5). The initial salvage rate of nonmaturing AVFs ranges in the literature from 74% to 97%.50–57 Numerous techniques have been used to improve fistula function, including venous and arterial angioplasty, stent placement, thrombectomy, venous branch ligation, and fistula superficialization.58–59 In an effort to increase the number of primary fistulas and expedite the use of a functional AVF, two new techniques have emerged, which include primary balloon angioplasty during fistula creation (in selected patients with small veins) and “balloon-assisted maturation” of immature AVFs by angioplasty.60,61 These techniques allow use of small or suboptimal veins to create functioning fistulas within 2 months and increase overall functional AVF rates to 90%.60,61
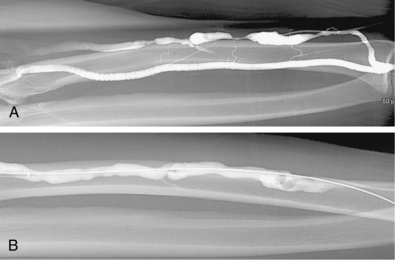
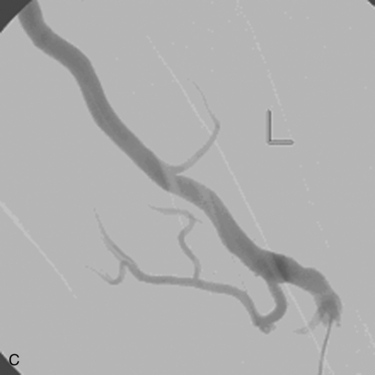
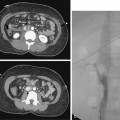
Venous anastomotic stenoses are the most common reason for malfunction of dialysis grafts. They usually occur in the vein within a few centimeters of the graft-vein anastomosis (Figs. 19-6 and 19-7). At times, multiple venous channels may overlap and conceal important stenosis. Several oblique views may be required to visualize the narrowing. NKF-KDOQI guidelines recommend treating stenoses greater than 50% only when there are concomitant clinical abnormalities and flow-rate reduction or pressure changes. After the stenosis is found, it is dilated with an appropriately sized angioplasty balloon catheter. Balloon diameter is generally 1 mm larger than the graft or 10% to 20% oversized compared to the adjacent normal vein. In the forearm and near the elbow, a 6- or 7-mm balloon typically is used initially. On occasion, veins may require 5- to 8-mm balloons. High-pressure balloons (burst pressures 20 to 30 mm Hg) often are required to dilate these lesions. Very high pressures (>20 atm) are more frequently needed in native fistulas.62 Some data may suggest longer inflation times are associated with less residual stenosis.62
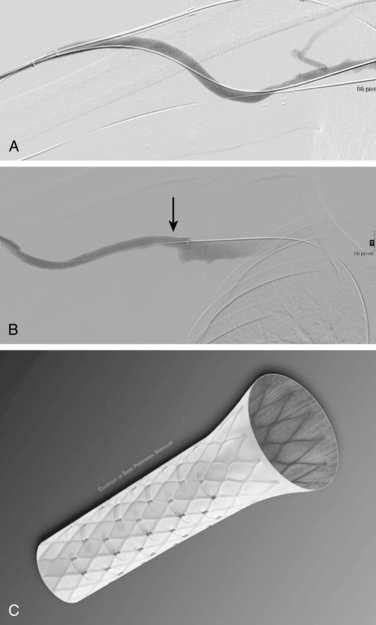
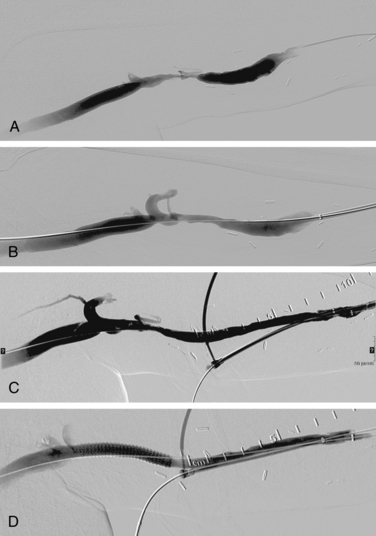
If the stenosis does not yield to balloon dilation (resistant stenosis), repeated inflations may be required to open the vessel. Multiple, prolonged inflations (e.g., 5 minutes) are thought to be useful in resistant lesions. Cutting balloons have been used for extremely resistant stenoses63–67 (Fig. 19-8). The devices have multiple microblades embedded in the balloon that incise the fibrotic vein wall and allow successful angioplasty to follow. There is no evidence that cutting balloons are superior to standard balloons for simple stenoses.68However, improved 6-month primary patency rates are noted for truly resistant lesions.69
In other cases, the balloon may completely inflate, but the stenosis remains at angiography following the inflation (elastic stenosis). Multiple (usually two) cycles of prolonged angioplasty (e.g., 5 minutes) can be effective for treatment of elastic stenoses. If the patient has minimal to no pain with the initial angioplasty, a larger balloon can be used. Cutting balloons have also been used to correct the elastic recoil. Regardless of the method, the goal of angioplasty is less than 30% residual stenosis after treatment, restoration of the thrill in the access, and successful dialysis.70
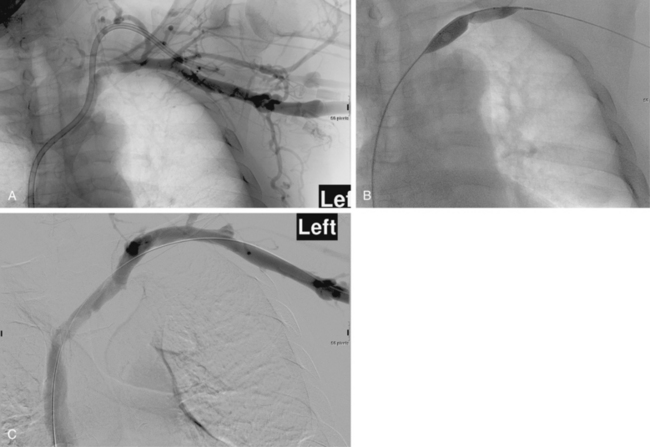
Stent placement has been used for venous stenoses, but the use of stents should be very selective. Indications for metal stents include surgically inaccessible stenoses that fail angioplasty, postangioplasty vein rupture not responsive to repeat balloon inflation, elastic recoil, and frequent or exuberant restenosis1 (Fig. 19-9). Stents may improve the immediate results of angioplasty, but their long-term benefit often is less favorable. Intimal hyperplasia commonly develops within the stent as well as at the ends of the stent. Veins that could be used for a new access should not be stented because that intervention limits potential revision. The safety of stent insertion at sites of bending or motion (e.g., the elbow joint) is debatable.
There are promising data concerning the use of stent grafts within dysfunctional grafts and fistulas (see Figs. 19-7 and 19-9). A covered stent may prevent or limit restenosis related to intimal hyperplasia.71 In a limited retrospective study, stent graft placement in dysfunctional autogenous hemodialysis fistulas was effective in preserving function and preventing access abandonment with patency exceeding angioplasty and uncovered stents.72 The new Flair (Bard Peripheral Vascular, Tempe, Ariz.) stent graft is designed to optimize flow when the graft and outflow vein have a diameter mismatch (see Fig. 19-7). The device is covered with ePTFE and includes a 4-mm flared end that extends into the outflow vein for improved flow dynamics. A recent randomized trial noted significantly better long term patency and freedom from repeat interventions than standard balloon angioplasty.36 Covered stents also allow rapid treatment of an acute venous rupture after angioplasty if balloon tamponade fails. 50,73–76
Arterial stenoses have been reported as being responsible for graft dysfunction in up to 28% of cases, although some series have noted an incidence of less than 15%.77–80 Construction of the dialysis access creates a low-resistance/high-flow circuit that may unmask a significant arterial inflow stenosis.77,80 The inflow artery and arterial anastomosis are visualized by direct injection at this site, manually compressing the mid-portion of the graft during injection, or inflating a balloon catheter with the end hole directed toward the anastomosis. Several projections may be required to adequately delineate the arterial inflow. If a significant arterial stenosis is identified, angioplasty usually is indicated. The lesion often can be reached through the access. Otherwise, a direct brachial (or common femoral) arterial puncture is necessary. In some cases, the arterial lesion is in the inflow vessels, so arteriography should be considered during the evaluation of a dysfunctional access, particularly if the patient has recurring graft dysfunction without an evident cause.77,81 Measuring flow and pressures during percutaneous procedures may lead to increased detection of arterial stenoses.77
Intragraft stenoses are relatively uncommon. These stenoses usually respond well to angioplasty (see Fig. 19-6). Balloon sizing in the graft should take into account that these grafts are most commonly 6 mm in diameter. Occasionally the grafts also are tapered at the arterial anastomosis (4 to 6 mm or 4 to 7 mm). In most cases a balloon that is 1 mm larger than the graft diameter is an appropriate size. If there is graft degeneration, it is particularly important that a larger balloon not be used because the graft can rupture.
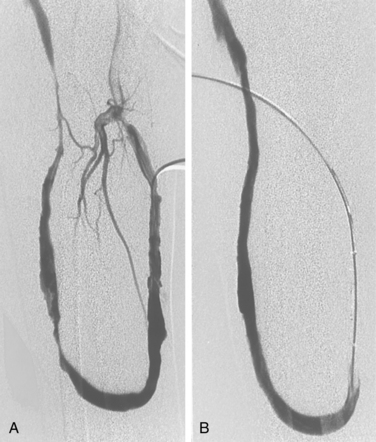
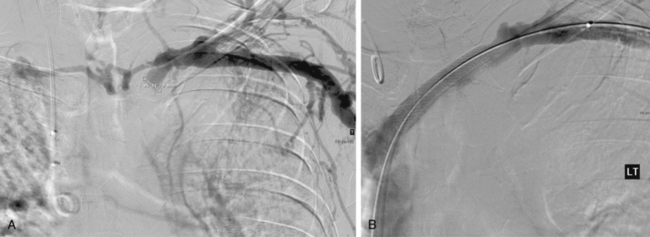
Central venous stenoses are an important, although less frequent, problem with hemodialysis grafts. Stenosis of the subclavian and brachiocephalic veins usually is caused by venous injury from prior dialysis catheters (Fig. 19-10). The importance of these obstructions is their potential to compromise future dialysis access sites and to cause significant arm swelling. Outflow stenoses should be treated with angioplasty only if they are considered to be responsible for graft dysfunction (see Fig. 19-10). If the lesion is not causing symptoms, it should be left alone. Angioplasty of symptomatic central venous stenoses greater than 50% have been associated with more rapid stenosis progression of the lesion.82 If a patient has a thrombosed graft, the most likely culprit is a stenosis at the venous anastomosis. Unless the outflow is thrombosed up to the level of the central vein, a central venous obstruction alone is probably not responsible for thrombosis. Near the shoulder and in the central veins, 10- to 12-mm balloons (or larger) may be necessary. The results of stent placement are not markedly better than those of balloon angioplasty in the central veins.83 The NKF-KDOQI and Society of Interventional Radiology guidelines recommend transluminal angioplasty as the preferred treatment for central vein stenosis, and stent placement is indicated only with elastic lesions, when stenosis recurs within 3 months, or with occluded vessels1,84 (Fig. 19-11). The stent needs to be carefully oversized to avoid migration.85–88 Balloon-expandable stents must be avoided at sites subject to extrinsic compression (e.g., subclavian vein running through the costoclavicular space). Stents should not be placed across the internal jugular or contralateral innominate veins, which may be needed for future dialysis catheters.
Results
Published series of nonthrombosed PTFE arteriovenous grafts have a reported 6-month primary (unassisted) patency of 40% to 50% after angioplasty.89–94 Repeated angioplasty enabled a primary assisted patency of 68% at 1 year and 51% at 2 years in one study. The secondary patency rate (allowing for intercurrent thrombolysis and angioplasty) was 82% at 1 year and 65% at 2 years in the same study. Although these patency rates seem low, they are comparable to results of operative treatment. Based on such reports, NKF-KDOQI endorses secondary patency rates after endovascular treatment for dysfunction of at least 70% at 1 year, 60% at 2 years, and 50% at 3 years.1 Primary unassisted patency (time from one intervention to any other intervention to maintain patency) following angioplasty of failing grafts should approach 50% at 6 months.1 The role of stent grafts for treatment of venous anastomotic graft stenoses has shown promise, with recent data demonstrating 6-month treatment site and total access circuit patency of 51% and 38% in the stent graft group and 23% and 20% in the balloon angioplasty group, respectively.36 In addition, freedom from subsequent interventions at 6 months was significantly greater in the stent graft group than in the balloon angioplasty group (32% vs. 16%). Longer-term data are unavailable, but stent grafts may have an important impact on future management of access grafts.
Clinical results for dialysis AVFs are variable. The initial salvage of nonmaturing AVFs ranges from 74% to 97% in the literature.50–57 One study of dysfunctional fistulas had a primary, assisted primary, and secondary patency rates in 53 dysfunctional fistulas after intervention at 3 months of 84% ± 5%, 88% ± 5%, and 90% ± 4%, respectively. At 6 months, the patency rates were 55% ± 8%, 80% ± 6%, and 82% ± 6%. At 12 months the rates were 26% ± 11%, 80% ± 6%, and 82% ± 6%.95 Turmel-Rodrigues reported a primary patency rate of 39% and a secondary patency rate of 79% at 12 months in dysfunctional forearm fistulas, and a rate of 57% in patients with upper arm fistulas.96 Others have reported a 1-year secondary patency rate of 64% and a 2-year secondary patency rate of 53%, with fewer procedures to keep the fistulas functioning in comparison with grafts.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree