CHAPTER 6 Thoracic aorta
Arteriography
Thoracic aortography usually is done through a femoral artery approach. In individuals with a history of catheterization-related cholesterol embolization, angiography is sometimes performed via the proximal left brachial artery to avoid recurrent showering of plaque fragments. Although catheter angiography is rarely required in patients with suspected traumatic aortic injury, in that situation the arch should be traversed with a guidewire. Advancing a formed pigtail catheter into a traumatic pseudoaneurysm can be lethal.1 A pigtail or similarly shaped catheter is placed about 2 cm above the aortic valve to avoid catheter recoil into the left ventricle during rapid contrast injection. A steep right posterior oblique projection is standard. Additional projections are obtained as needed.
Anatomy
Development
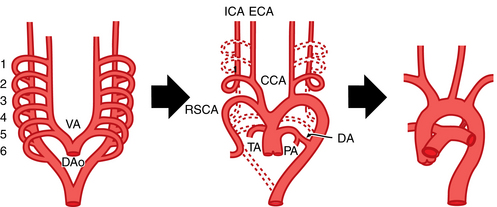
In the fetus, two paired blood vessels (dorsal and ventral aortae) form in the chest.2 The ventral aortae merge into the aortic sac, which forms parts of the ascending aorta, aortic arch, and pulmonary trunk. The left dorsal aorta becomes the descending thoracic aorta. Six pairs of branchial arches connect these two major vascular channels (Fig. 6-1 and Table 6-1). The normal left aortic arch persists while the right arch involutes beyond the origin of the right subclavian artery. The remaining portion of the right arch becomes the brachiocephalic (innominate) artery.
Table 6-1 Fate of Embryologic Aortic and Branchial Vessels
| Embryologic Vessel | Neonatal Vessel |
|---|---|
| Ventral aorta (upper) | External carotid artery |
| Dorsal aorta (upper) | Internal carotid artery |
| Branchial arch 1 | Maxillary artery |
| Branchial arch 2 | Hyoid and stapedial arteries |
| Branchial arch 3 | Carotid arteries |
| Branchial arch 4 | Aortic arch |
| Branchial arch 5 | Involutes |
| Branchial arch 6 | Pulmonary artery, ductus arteriosus |
| 7th intersegmental artery | Subclavian artery |
Normal anatomy
The aortic valve is composed of three leaflets that form the three sinuses of Valsalva: right, left, and posterior or noncoronary3 (Fig. 6-2). The right and left coronary arteries arise from their respective sinuses. Beyond the sinus segment, the ascending aorta courses anteriorly and superiorly. This region is enclosed within the fibrous pericardium. In adults, its mean diameter at end diastole is 2.8 cm.4 The aorta then passes over the main pulmonary artery and left main stem bronchus. The brachiocephalic, left common carotid, and left subclavian arteries take off in turn from the upper surface of the aortic arch.
Just beyond the origin of the left subclavian artery, the aorta narrows at the aortic isthmus. It then widens slightly beyond this point at the aortic spindle. The ligamentum arteriosum, a remnant of the ductus arteriosus, tethers the aorta to the left pulmonary artery at the isthmus. In some cases, a shallow, smooth, symmetric outpouching persists: the ductus diverticulum, or “ductus bump” (Fig. 6-3).
The descending thoracic aorta runs in front of the spine. In adults, its mean diameter at end diastole is about 2.2 cm.4 Nine pairs of posterior intercostal arteries (levels 3 through 11) exit the descending aorta. The first and second posterior intercostal arteries are branches of the superior intercostal artery, which arises from the costocervical branch of the subclavian artery (see Chapter 9). The subcostal arteries take off at the level of the 12th ribs. One or more bronchial arteries supply each lung and originate as separate branches or bronchointercostal trunks typically at the fourth to sixth thoracic level (see Chapter 14). Numerous small vessels that supply the chest wall, mediastinum, esophagus, spinal cord, and pericardium are rarely seen during aortography.
Variant anatomy (online cases 1 and 73)
Congenital anomalies of the thoracic aorta can be explained by abnormal evolution of the dorsal and ventral aortae and the branchial arches using the hypothetical double aortic arch system proposed by Edwards5 (Box 6-1). The aorta, pulmonary artery, and anomalous branches may form vascular rings that compress the trachea or esophagus. Aortic arch variants often are associated with congenital cardiac anomalies.6
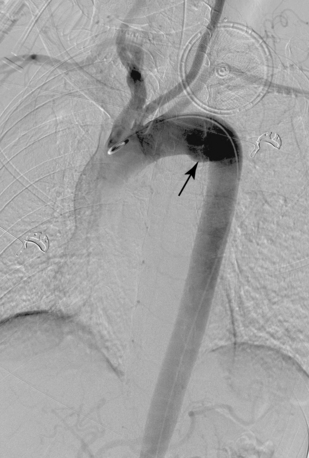
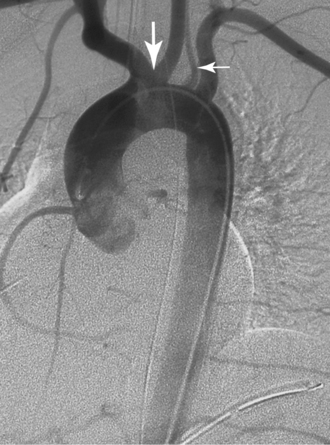
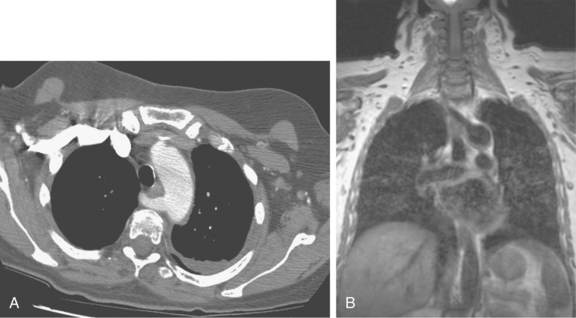
A common origin of the brachiocephalic and left common carotid arteries or origin of the left common carotid artery from the root of the brachiocephalic artery (bovine arch) is reported in 3% to 29% of the population based on autopsy and imaging studies; the true figure is probably closer to 10% to 15%7,8 (Fig. 6-4). The origin of the term bovine arch is disputed. It may reflect the configuration of the vessels (like a bull’s horn); this anatomy is not typical in cattle. Takeoff of the left vertebral artery directly from the arch is an uncommon variant (see Fig. 6-4). Separate origin of the right subclavian and common carotid arteries from the arch is rare.
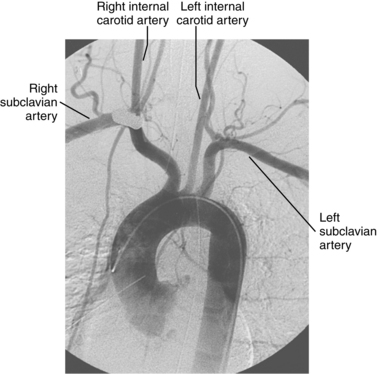
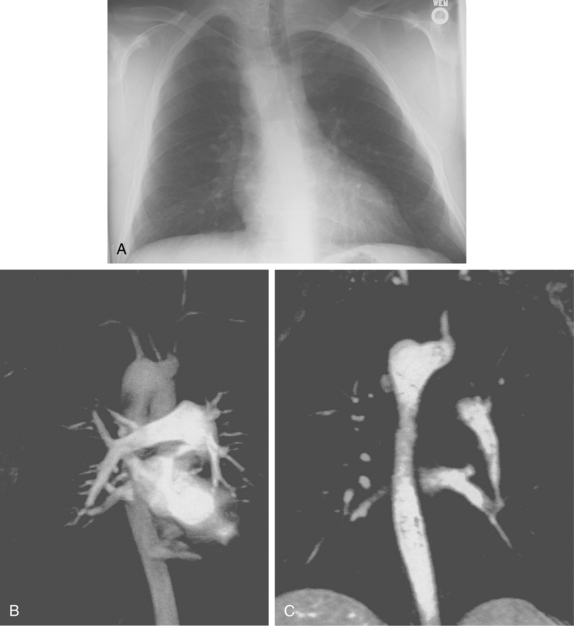
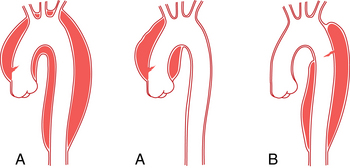
An aberrant right subclavian artery is the most common aortic arch malformation of clinical significance, with a reported frequency of 0.4% to 2.3%.9 This anomaly results from involution of the embryonic aortic segment between the right subclavian and right common carotid arteries. The right subclavian artery becomes the last branch of the aortic arch (Fig. 6-5). It usually passes behind the trachea and esophagus to reach the right side of the chest. The vessel may indent the posterior surface of the esophagus, but patients rarely have symptoms.10 The aberrant artery often originates from a dilated portion of the most distal remnant of the right aortic arch, the so-called diverticulum of Kommerell.
Right aortic arch with aberrant left subclavian artery is the most common of the several types of right aortic arch, occurring in about 0.1% of the population.11 The root cause is involution of the left aortic arch between the left common and left subclavian arteries with persistence of the embryologic right arch. The order of aortic arch branching is therefore left carotid artery, right carotid artery, right subclavian artery, and left subclavian artery. The arch passes over the right main stem bronchus and descends on either side of the spine (Fig. 6-6). Despite the presence of a vascular ring, respiratory and esophageal symptoms are infrequent. Few of these individuals have associated congenital heart disease.
Right aortic arch with mirror-image branching imposes a branch order of left brachiocephalic artery, right common carotid artery, and right subclavian artery.12 The descending thoracic aorta is virtually always to the right of the spine. Some form of congenital heart disease is found in 90% to 95% of patients, including tetralogy of Fallot, truncus arteriosus, double-outlet right ventricle, and transposition of the great vessels. About one third of patients with tetralogy of Fallot have a right aortic arch, usually of this type. Although the vascular ring created by the right arch and ligamentum arteriosum produces tracheal or esophageal compression, patients usually have no symptoms.
Major disorders
Atherosclerosis
Atherosclerosis is the most common disease affecting the thoracic aorta, but usually it is silent. Plaques cause symptoms when fragments embolize into the brain or peripheral circulation. Emboli usually are released from “protruding” plaques with a mobile component that is probably overlying thrombus.13 Most of these symptomatic lesions are found in the aortic arch and descending thoracic aorta. About one third of patients with such atheromas suffer distal embolization within 1 year of detection. Cholesterol crystals may shower into the distal circulation (spontaneously or after aortic manipulation) and result in renal insufficiency, bowel ischemia, or blue toe syndrome.14
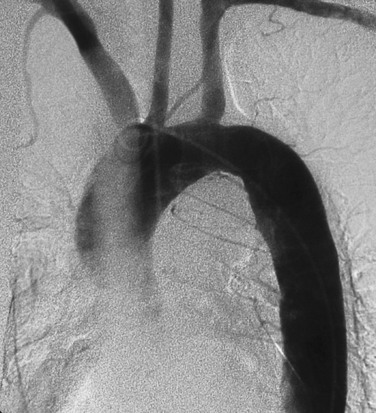
Suspected thoracic aortic atherosclerosis in patients with unexplained stroke or peripheral embolization is studied by transesophageal echocardiography (TEE), computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) imaging.13,15 The disease produces an irregular contour to the aortic lumen, typically in the arch or distal aorta (Fig. 6-7). Rarely, ulcerated plaque penetrates into the aortic wall (see later discussion). Other causes for narrowing of the aortic lumen include dissection (with encroachment by the false lumen), arteritis, and noninflammatory vasculopathies (see Box 1-10).
Degenerative aneurysms (online case 96)
Etiology and natural history
Thoracic aortic aneurysms (TAAs) and pseudoaneurysms have many causes16–18 (Box 6-2). The classic features of several types are summarized in Table 6-2. The pathologic and imaging features of the uncommon forms are considered in later sections of this chapter.
Table 6-2 Classic Appearance of Thoracic Aortic Aneurysms
| Type | Features |
|---|---|
| Degenerative | Fusiform aneurysm of the descending aorta with atherosclerotic changes |
| Post-traumatic | Saccular pseudoaneurysm at the aortic isthmus |
| Postoperative | Saccular aneurysm at stent-graft termination, suture line, or cannulation site |
| Bacterial | Saccular, eccentric aneurysm at an unusual site |
| Marfan syndrome | Aneurysm of aortic root and tubular segment (sinotubular or annuloaortic ectasia) |
| Syphilitic | Saccular ascending aortic or arch aneurysm sparing the aortic root |
| Takayasu arteritis | Wall thickening and enhancement (early) Diffuse fusiform dilation of the aorta with branch stenoses or occlusions (late) |
| Post-stenotic | Dilation beyond an obstruction (e.g., aortic stenosis, coarctation) |
About 75% of TAAs are degenerative (“atherosclerotic”) aneurysms that form because of weakening of the aortic wall by enhanced proteolytic activity or hemodynamic forces that cause pathologic remodeling.16–18 Most degenerative thoracic aneurysms involve the descending segment; about 5% are thoracoabdominal.19 The most common cause of isolatedascending aortic aneurysm is intrinsic medial degeneration20 (e.g., Marfan syndrome, see Chapter 1).
Compared with abdominal aortic aneurysms, the natural history of thoracic aneurysms is less well understood. The most feared complication is rupture. Based on Laplace’s law (wall stress = pressure × radius), the strongest predictor of rupture is aneurysm size. Less important risk factors for TAA rupture are advanced age, hypertension, chronic lung disease, and renal failure.21 The likelihood of rupture is low if the aneurysm is less than 5 cm in diameter, unless the rate of expansion exceeds about 1 cm/yr. After a TAA reaches 5 to 6 cm in diameter, the risk of subsequent rupture increases substantially (i.e., 5-year mortality between 38% and 64%).22–25 As a rule, such patients should undergo elective aneurysm repair.
Clinical features
Most affected patients are older and asymptomatic at the time the aneurysm is discovered on a routine chest radiograph or CT scan obtained for unrelated reasons. Unusual complaints related to compression of adjacent organs include dysphagia, respiratory distress, superior vena cava syndrome, and hoarseness from impingement of the recurrent laryngeal nerve. Some individuals with intact aneurysms do complain of chest or back pain. However, symptoms are expected when the aneurysm ruptures. They include chest pain, hypotension, massive hemoptysis (from aortobronchial fistula), hematemesis (from the even rarer aortoesophageal fistula), and dyspnea from leakage into the pleural space.
Imaging
Transesophageal echocardiography
TEE can both detect and size TAA.26 However, TEE is less valuable for aneurysm staging, evaluation of adjacent structures, or postoperative surveillance.
Computed tomography
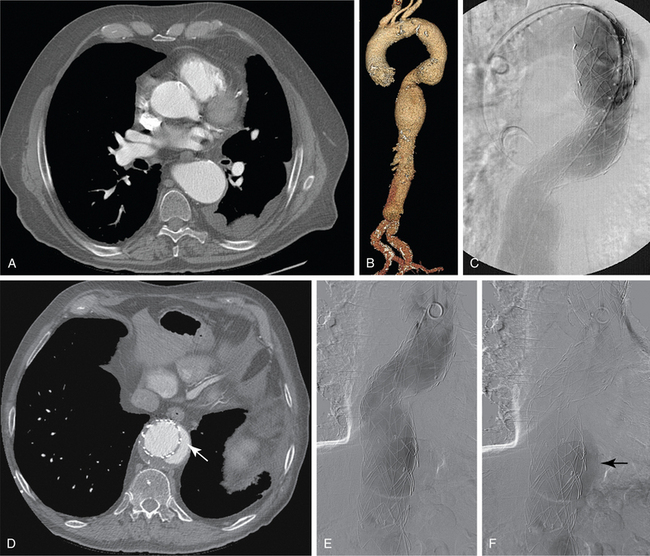
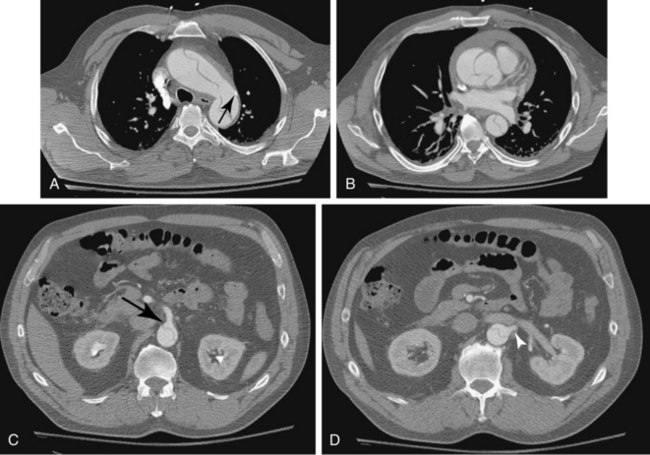
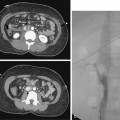
CT is the standard tool for the diagnosis of suspected TAA, surveillance of known aneurysms, and detection of sac rupture.27 Aneurysm diameter and length, presence of mural thrombus, and associated atherosclerotic disease are well depicted by CT (Fig. 6-8). A high-density “crescent” in the mural thrombus may be a harbinger of contained or imminent rupture. Multiplanar reformatted and shaded-surface display images provide exquisite views of the aneurysm, its relationship to adjacent structures (e.g., compression of the pulmonary artery), and involvement of arch vessels.16,28
Magnetic resonance imaging
MRI is probably the best modality for studying TAA in patients in stable condition, although it is not effective after endograft repair. Gadolinium-enhanced MR angiography characterizes the entire extent of the aneurysm, its effect on neighboring organs, branch vessel involvement, the aortic valve, and the heart.6,29 Several features can sometimes differentiate degenerative aneurysms from other types (see Table 6-2).
Treatment
Surgical therapy
The traditional treatment for TAAs is operative.30 As a rule, they are repaired when the diameter exceeds 5 to 6 cm or the patient is symptomatic (see earlier discussion). An interposition graft is placed by end-to-end anastomosis of a synthetic conduit between the excised ends of the aorta. Alternatively, the diseased portion of the aorta is wrapped around the graft material sewn to the aorta at both ends as an inclusion graft. With proximal aortic disease, an elephant trunk technique may be employed.31 The ascending aortic and arch segments are replaced with graft material and branch vessels connected into it; the distal free end of the graft floats in the descending thoracic aorta. At a later stage, the surgeon creates a permanent anastomosis to the normal lower descending thoracic aorta.
Major complications of operative TAA repair include stroke, myocardial infarction, and renal failure. Paraplegia is a rather unique and devastating event caused by exclusion of branches to the spinal artery. It is more frequent with repair of thoracoabdominal aneurysms. The frequency of spinal cord injury is well under 10% with the use of intraoperative maneuvers such as distal perfusion, hypothermia, and monitoring of evoked potentials.32,33 Preoperative spinal arteriography may be useful in this setting to allow reimplantation of major spinal artery branches.34 In contemporary series, the overall mortality rate for elective repair is still substantial but far better than for operation with rupture (3% to 8% vs. 22%).32,33
Endovascular therapy
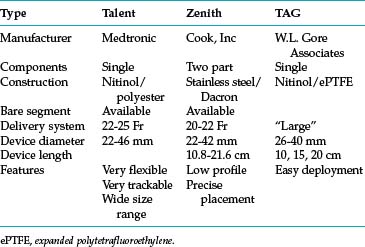
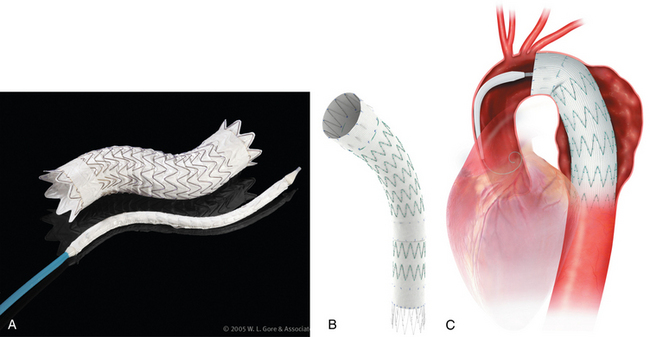
Thoracic endovascular aneurysm repair (TEVAR) with stent grafts is an attractive alternative to open surgery for selected patients with stable or ruptured degenerative TAAs.35–37 Several devices are approved by the U.S. Food and Drug Administration for treatment of this particular disorder (Table 6-3 and Fig. 6-9). The choice of stent graft is based on patient factors, aneurysm characteristics, and operator preference. The TAG device can be inserted rapidly but with somewhat less precision than other endografts. The Talent device (and the newer iteration Valiant stent) allows accurate deployment and is easier to use with severe aortic angulation or iliac artery access tortuosity. Finally, the Zenith device has the lowest profile (at present) and permits the most accurate deployment.
Technical success and immediate aneurysm thrombosis is noted in more than 90% of cases with very low mortality rates38 (see Fig. 6-8). Unlike endograft treatment of abdominal aortic aneurysms (see Chapter 7), the most common type of endoleak with TAA (up to 30% of cases) is a type I defect at an attachment site.39,40 In this situation, additional interventions (further balloon treatment, insertion of additional stents or stent grafts) are usually necessary. Uncommon early complications include stroke and paraplegia from exclusion of side branches feeding the spinal cord.41 Late complications involve proximal and distal endoleaks, aneurysm growth at the edges of the device, stent graft movement, kinking or fracture, and aneurysm rupture.
The choice between open and endovascular repair of TAA is made difficult by the almost complete lack of randomized, controlled trials comparing the two approaches.36,37 Two large, prospective, single-arm trials with historical controls noted significantly lower early morbidity and mortality and paraplegia rates with TEVAR.38,42 Reported outcomes were technical success 96%, major adverse events 41%, 30-day mortality 2.1%, paraplegia 1.5%, stroke 3.6%, aneurysm rupture at 1 year 0.5%, migration 4%, and endoleak 12.2%. One study observed better 1-year mortality with TEVAR, whereas the other showed no difference at 2 years. A recent metaanalysis calculated a significantly lower incidence of neurologic injury and perioperative mortality with TEVAR.
Dissection (online cases 13 and 52)
Etiology
A dissection is a longitudinal split in the media of the aortic wall. In most cases, an intimal tear connects this medial channel with the aortic lumen. Dissections confined to the aortic wall are intramural hematomas43 (see later discussion).
The pathogenesis of aortic dissection is not entirely understood.44 Dissection is usually attributed to hemodynamic stresses such as long-standing hypertension or to abnormalities of the media (some of which are congenital)45 (Box 6-3). In the former group, the pathologic basis for dissection is unclear; only mild medial degeneration is seen histologically. In the latter group, degeneration of medial smooth muscle, elastin, or collagen matrix can be identified. The reason that pregnant women are at greater risk for dissection is unknown, although hemodynamic factors and hormonal effects have been implicated. Illicit drug use (particularly cocaine and methamphetamines) is being recognized as an increasingly frequent cause of acute dissection.46 Occasionally, aortic dissection is caused by trauma (e.g., deceleration injury, catheterization procedures).47
The location and extent of the dissection has important implications for treatment and prognosis. The DeBakey and Stanford classifications are illustrated in Figure 6-10. Stanford type A dissections, which usually begin with a tear just above the aortic valve and always involve the ascending aorta, account for about two thirds of cases.48Type B dissections, which usually begin with a tear just beyond the origin of the left subclavian artery and only involve the descending aorta, account for the remaining cases. Sometimes, a dissection that begins at this site extends distally and proximally toward the aortic valve.
Natural history
Early complications of aortic dissection are the result of complete rupture (often at the site of the intimal tear) or ischemia. Advances in diagnosis and treatment have improved outcomes, but even in the best of hands the mortality rate still exceeds 18% to 25%.49,50 Rupture, which often is fatal, can occur from the ascending aorta into the pericardium, from the aortic arch into the mediastinum, or from the descending aorta into the mediastinum or left pleural space. In about half of patients with type A dissection, aortic regurgitation follows damage to the aortic root.
If the intimal flap obstructs a major aortic branch or flow is diminished in the true or false channel supplying an organ, ischemia can result (so-called malperfusion syndrome). In most of these cases, the obstruction is “dynamic” (prolapse of flap into vessel orifice or inadequate flow in the lumen feeding the tissue).49 In this situation, some type of fenestration to increase blood pressure in the stagnant lumen is indicated (see later discussion). Less often, “static” obstruction from extension of the flap into a major branch is responsible for target organ ischemia. In this event, direct recanalization by stent insertion or operative bypass is necessary.
Over time, untreated dissections can persist (i.e., chronic dissection), enlarge, thrombose, or rupture. A dissection is consider chronic if it is present for more than 2 weeks. Late complications of untreated chronic dissections include aneurysm formation and delayed ischemic events. Even with appropriate medical therapy, 25% to 40% of surviving patients will go on to aneurysm formation.44
Clinical features
Aortic dissection is one of several diseases included in the acute aortic syndromes which feature the constellation of chest pain and chronic hypertension51 (see later discussion). These disorders are easily confused with the acute coronary syndromes. In modern series, men suffer aortic dissections about four or five times as frequently as women. Most patients are older than 50 years and have one or more risk factors for dissection (see Box 6-3). Hypertension is present in about three fourths of patients and is even more common in patients with type B dissection. The classic presentation is the sudden onset of searing chest or back pain. Patients also may present with a stroke, renal failure or renovascular hypertension, mesenteric ischemia, lower extremity ischemia, or even paraplegia from obstruction of spinal artery branches of the descending aorta.
Imaging
Chest radiography
Plain radiographs of the chest may point to the diagnosis of aortic dissection. The most common findings are an increase in size of the thoracic aorta, a double aortic arch contour, widening of the mediastinal silhouette, pleural effusion, and tracheal shift.52 A completely normal chest radiograph does not exclude the diagnosis.
Computed tomography
CT is widely used for evaluation of acute aortic dissection.16,52,53 CT is readily available, relatively noninvasive, and accommodates critically ill patients.
Scans reveal an intimal flap separating the two lumens, often with different enhancement patterns (Fig. 6-11). Other possible findings are dilation of the aorta, compression of the true lumen by the false lumen, thrombosis of the false lumen, mediastinal hematoma, and pleural or pericardial effusion. A localized dissection must be differentiated from an intramural hematoma or a penetrating aortic ulcer (see later discussion).