CHAPTER 7 Abdominal Aorta
ANATOMY
Development
In the embryo, the abdominal aorta is formed from the right and left dorsal aortae.1 Numerous ventral splanchnic branches of the aorta supply the primitive digestive tract. These channels are eventually reduced to the celiac, superior mesenteric, and inferior mesenteric arteries. Lateral splanchnic branches supply organs arising from the mesonephric ridge, giving rise to the inferior phrenic, adrenal, renal, and gonadal arteries. Four sets of somatic arterial branches evolve into the intersegmental lumbar arteries.
Normal Anatomy
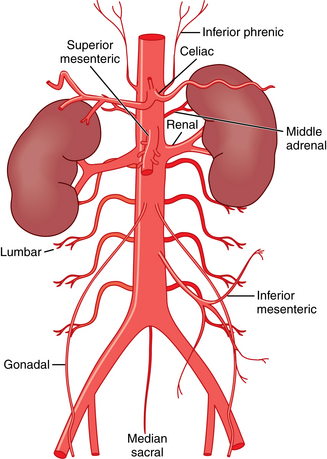
The abdominal aorta begins at the diaphragmatic hiatus.2 The vessel runs in front of the spine and to the left of the inferior vena cava (IVC) until it bifurcates into the common iliac arteries at about the L4 vertebra. The normal caliber of the abdominal aorta increases with age; at the renal hila, its mean diameter varies from about 1.5 cm in women in the fourth decade of life to about 2 cm in men in the eighth decade.3,4
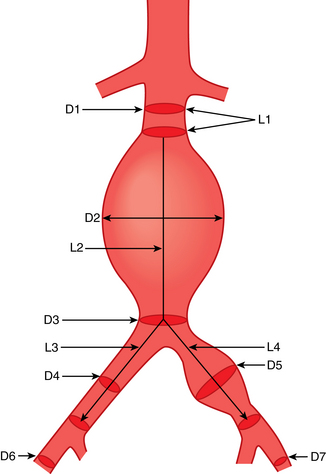
The abdominal aorta has three ventral branches (Figs. 7-1 and 7-2). The celiac artery arises at the T12-L1 level. It can initially take a forward, upward, or dowward course. The superior mesenteric artery (SMA) takes off at the L1-L2 level about 1 cm below the celiac axis. The inferior mesenteric artery (IMA) originates at the L3-L4 level, typically on the left anterolateral surface of the aorta.
The paired inferior phrenic arteries classically originate at or just above the celiac artery. They course superolaterally toward the central tendon of the hemidiaphragms, where they bifurcate. Paired middle adrenal arteries take off from the aorta adjacent to the SMA to provide partial blood supply to the adrenal glands. The renal arteries originate just below or at the level of the SMA. The gonadal (testicular or ovarian) arteries are paired vessels off the anterolateral surface of the aorta just below the main renal arteries.
Four pairs of lumbar arteries exit the posterolateral aspect of the aorta. There are extensive anastomoses between these vessels, the lower intercostal arteries (superiorly), and the iliolumbar, deep iliac circumflex, and gluteal vessels (inferiorly). The median sacral artery takes off posteriorly just above the aortic bifurcation and descends toward the coccyx.
Variant Anatomy
Congenital anomalies of the abdominal aorta are rare. However, anomalies of the primary branches are common (see Chapters 10 and 11). Multiple renal arteries are present in about 25% to 35% of the population.5–7 Although most accessory arteries are found just below the main renal artery, they may arise above the main trunk or as far distally as the iliac artery (Fig. 7-3).
Persistence of the embryonic ventral connection between the celiac artery and SMA with regression of the origin of one of these vessels leads to the rare celiomesenteric artery8 (see Fig. 11-10). The hepatic, left gastric, or splenic arteries may have a separate origin from the aorta (Fig. 7-4). The inferior phrenic arteries can exit the top of the celiac (or rarely a renal) artery or form a common trunk from the aorta.9,10 Rarely, a pair of lumbar arteries arise as a single branch from the posterior aspect of the aorta.
Collateral Circulation
With severe narrowing or complete occlusion of the abdominal aorta, several parietal and visceral collateral pathways divert blood flow around the obstructed segment. The chief parietal collateral routes are from the intercostal, subcostal, and lumbar arteries to the iliolumbar, lateral sacral, and superior gluteal branches of the internal iliac artery and to the deep and superficial iliac circumflex branches of the external iliac artery (Fig. 7-5). With the latter system, blood flows through the internal iliac artery to feed the external iliac artery in a retrograde direction. Interconnections between the lumbar arteries help to support this collateral network. A pathway also exists from the superior epigastric artery (terminal branch of the internal thoracic artery) to the inferior epigastric artery, which joins the common femoral artery.
The SMA and IMA are critical visceral conduits when the abdominal aorta is obstructed. With occlusion of the aorta above the level of the IMA, the SMA feeds the IMA through the middle colic and marginal arteries, which then connect with branches of the internal iliac artery through the rectal network (see Fig. 11-15). With occlusion of the abdominal aorta below the IMA origin, the rectal collateral network (between the superior rectal branch of the IMA and the middle and inferior rectal branches of the internal iliac artery) may be an important communication (see Fig 7-5). In cases of short-segment aortic stenosis involving the SMA, the so-called meandering mesenteric artery can fill the SMA from the IMA circulation11 (see Fig. 11-16).
MAJOR DISORDERS
Atherosclerosis
Atherosclerosis is the most common disease affecting the abdominal aorta (Fig. 7-6). This condition is considered as part of lower extremity peripheral vascular disease in Chapter 8.
Degenerative Abdominal Aortic Aneurysms (Online Cases 14 and 65)
Etiology
An arterial aneurysm is defined as a localized dilation of the vessel by 50% or more of its normal diameter.12,13 By general agreement, an abdominal aortic aneurysm (AAA) is present in an adult when the wall-to-wall diameter of the infrarenal aorta exceeds 3 cm.14 Most AAAs are infrarenal; in large series, up to 15% are suprarenal or juxtarenal (extending ≤1 cm from the renal arteries).15–17 More than 20% of abdominal aneurysms extend into one or both common iliac arteries. The incidence of AAA has risen significantly during the last half century, and this phenomenon cannot be attributed entirely to earlier detection through screening or aging of the population.18,19
The vast majority of AAA are degenerative in nature. Although the strong association between abdominal aneurysms and atherosclerosis is well recognized, causation has not been proven.20–22 Degenerative AAAs form because of genetic predisposition, immune-related inflammatory damage to the vessel wall, weakening of components of the media, and hemodynamic forces that produce remodeling.20,23–25 The predilection for the infrarenal segment is possibly a consequence of reduced elastin content (relative to the suprarenal aorta) and a variety of hemodynamic factors. Although most AAAs are degenerative, a small percentage of patients develop aortic aneurysms for other reasons26–28 (Box 7-1).
Natural History
The most common and feared complication of AAA is rupture, the likelihood of which is governed by Laplace’s law (wall stress = pressure × radius). Rupture is thus mainly a function of aneurysm size.29 Although the diameter of a typical AAA will grow by 1 to 4 mm per year, the rate of expansion is unpredictable in a particular patient, and the growth rate is not completely linear.30,31 Expansion accelerates as the aneurysm gets bigger, even though periods of quiescence may occur. The rupture rate for AAAs that are 4 to 5.5 cm in diameter is about 0.7% to 1% per year.32 Elective repair (open or endovascular) of smaller asymptomatic aneurysms (<5.5 cm in diameter) does not appear to be beneficial relative to the attendant risks if strict imaging surveillance is maintained32–37 (see later discussion). The risk of AAA rupture increases substantially when the diameter is greater than 5 cm; for example, the 5-year risk of rupture of untreated AAAs of 5.5 cm or larger is 25% to 50%.31,32,34
Before the advent of endovascular therapy, AAA rupture was fatal in up to 90% of individuals.38 More than 60% of such patients did not survive to reach medical care, and the mortality rate with emergent open surgery can exceed 75%.38–40 When rupture occurs, the tear usually starts in the posterior or lateral wall of the aorta, with bleeding into the retroperitoneal space. Less often, the rupture occurs on the anterior wall, sometimes leading to hemorrhage into the peritoneal cavity.
Other sequelae of AAA include distal embolization, thrombosis, and aortocaval fistula. The latter complication occurs in fewer than 1% of untreated patients with AAA.41
Clinical Features
AAA is largely a disease of older adults and is strikingly more common in men than in women (approximately 6:1).39,42 The other strong risk factors for AAA are smoking and family history. Additional associations include hypertension, coronary and peripheral arterial disease, and chronic obstructive pulmonary disease.
Most patients are asymptomatic at the time the aneurysm is detected, usually by imaging studies obtained for screening or unrelated problems. A rapidly expanding aneurysm may produce abdominal or back pain. Less than one fourth of affected individuals present with aneurysm rupture, which is suggested by the triad of abdominal or back pain, a pulsatile abdominal mass, and hypotension. Occasionally, patients have symptoms caused by extrinsic compression of adjacent organs, aneurysm thrombosis, distal embolization, or dissection. The rare patient with an aortocaval fistula may exhibit pain, a pulsatile abdominal mass, continuous abdominal bruit or thrill, congestive heart failure, lower extremity edema, or hematuria.
Imaging
Sonography
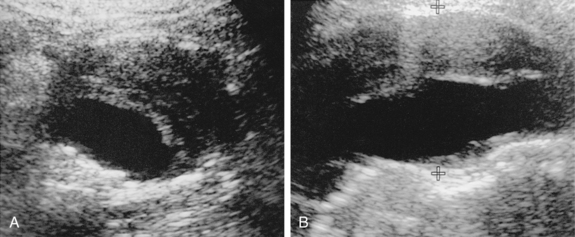
Ultrasound (US) is the chief tool for detecting, sizing, and monitoring AAAs35 (Fig. 7-7). The technique is almost 100% sensitive for aneurysm diagnosis. Body habitus or overlying structures (e.g., bowel gas) makes the test useless in some individuals. There is strong evidence that US screening programs in populations at risk will reduce overall mortality attributable to this lethal disease.35,37 Sonography also is highly accurate in determining aneurysm size, the presence of mural thrombus, and associated abdominal pathology. US is less accurate in assessment of the cranial and caudal extent of the aneurysm, the relationship to and number of renal arteries, and the presence of iliofemoral arterial disease.
Computed Tomography
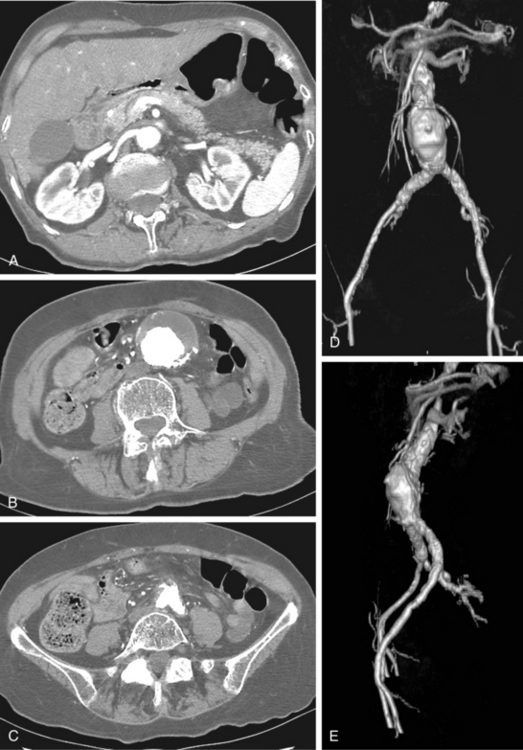
Computed tomography angiography (CTA) is the principal modality for preprocedure and postprocedure assessment of patients with AAA43–46 (Box 7-2). CT also is useful in following aneurysm growth in patients who cannot be imaged adequately with sonography. In those with underlying renal dysfunction, the need for iodinated contrast material is a major drawback of CTA.
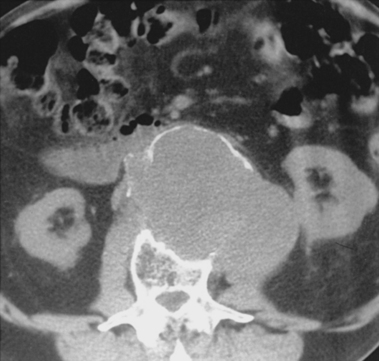
The typical AAA is a fusiform dilation of the infrarenal abdominal aorta (Fig. 7-8). Intimal calcification and mural thrombus are common. If the aneurysm is saccular or eccentric without calcification or adjacent aortic disease, or is present in a young patient, uncommon causes should be considered (see Box 7-1).
CTA is the best means for evaluating patients with suspected AAA rupture.45,47–49 Acute aneurysm rupture produces strandlike soft tissue density with high attenuation in the retroperitoneum adjacent to the aneurysm (often posteriorly) (Fig. 7-9). It may be difficult to pinpoint the exact site of leak. Several CT findings have been described as suggestive of impending aortic rupture, including a high-attenuation crescent sign within or between the luminal thrombus and the aortic wall.
Other entities can mimic acute aneurysm leak, including chronic rupture, perianeurysmal fibrosis from an inflammatory aneurysm (see later), lymphadenopathy, unopacified duodenum, and spontaneous retroperitoneal hemorrhage (Fig. 7-10).
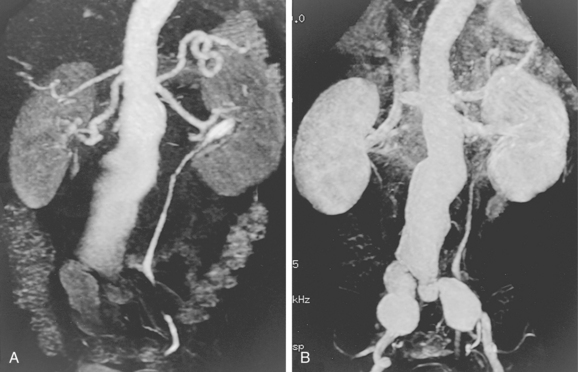
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is used in preprocedure and postprocedure assessment of some individuals with AAA50–52 (Fig. 7-11). The diagnosis of aortocaval fistula is usually made by CT or MR angiography (Fig. 7-12). The communication most frequently develops between the distal aorta and IVC. In the absence of AAA, aortocaval fistula is either spontaneous or secondary to infection or trauma.53,54 Stent grafts have been used to manage these rare lesions.55
Treatment
All patients with small AAAs should undergo routine imaging surveillance to follow growth. There is modest evidence that doxycycline (perhaps acting as an antiinflammatory agent) and coenzyme A reductase inhibitors (statins) will slow the progression of AAA expansion.56 The sole purpose of invasive AAA intervention is to prevent rupture.
Patient Selection
There is general agreement among vascular interventionalists that all AAA larger than 5 cm in transverse wall-to-wall diameter should be repaired electively and that smaller asymptomatic aneurysms be routinely followed by imaging studies.33–37 Smaller AAAs are considered for repair if they are symptomatic (causing pain, tenderness, or distal embolization) or enlarging rapidly (>5 mm/6 months).
Of course, the benefits of watchful waiting with imaging surveillance in a particular individual must be weighed against the operative mortality rate for elective open or endovascular AAA repair (≤5%) and the high mortality rate associated with ruptured AAAs (50% to 75%).36,39 Risk factors for rupture include female gender, advanced age, and continued smoking. The indications for open versus endovascular repair are evolving but are based primarily on anatomic factors, comorbid conditions, and patient/physician preference. Endovascular AAA repair (EVAR) with a stent graft should certainly be considered in patients with advanced age, significant operative risk, or a hostile abdomen.57 EVAR has been used also in selected patients with acute rupture with promising results.58,59 Frankly, however, the enthusiasm for EVAR is partly driven by patient demand for a less invasive and morbid solution to this potentially life-threatening condition.
Open Surgical Repair
Imaging
Before operation, several imaging features are particularly important to the surgeon (see Box 7-2). The relationship of the neck of the aneurysm to the renal and mesenteric arteries is critical because it influences the surgical approach, placement of the proximal aortic clamp, and the need for arterial reconstruction. For example, a juxtarenal AAA necessitates suprarenal aortic cross-clamping. Extension of the aneurysm into the iliac arteries or occlusive iliofemoral disease requires placement of a bifurcated graft.
Technique
The aneurysm is approached through an abdominal or retroperitoneal incision.60 Synthetic graft material is used to bypass the aneurysm. Even when the aneurysm is located well below the renal arteries, the proximal end-to-end anastomosis is made near the renal arteries to prevent recurrent disease in the infrarenal aorta. If the aneurysm ends above the bifurcation and the common iliac arteries are relatively disease-free, a tube graft to the aortic bifurcation may be inserted. Otherwise, a bifurcated graft to the common femoral (or iliac) arteries is placed. Reimplantation of the IMA is performed if collateral flow appears insufficient to maintain viability of the left colon. The aneurysm sac is then wrapped around the graft to avoid contact with the bowel, which can lead to infection or fistula formation. Open AAA repair is a major operation, leading to several days in an intensive care unit, a hospital stay of 5 to 10 days, and up to 2 months for recovery.
Complications
Graft infection occurs after less than 2% of open AAA repair procedures.35 Infections in the groin often are symptomatic and may be treated in some cases with intravenous antibiotics and debridement. Abdominal or pelvic infections may be relatively silent or masquerade as other disease. Suspected graft infection usually is evaluated by CT; radionuclide white blood cell scanning plays a secondary role. Signs of infection on CT include fluid density adjacent to or air within the graft.61 These findings may not be distinguishable from the normal appearance of the postoperative graft 2 to 3 months after operation. Infections generally require graft takedown and extraanatomic bypass. Recently, however, primary conduit replacement or insertion of cryopreserved allograft have been studied.62,63
Paraanastomotic aneurysm is infrequent in the short term but afflicts up to one third of open surgical repair patients at 15 years afterward.35 An infectious etiology should always be considered. They occur more frequently at the distal anastomosis than at the aortic end of the graft (see Fig. 8-44). Pseudoaneurysms can lead to graft thrombosis, rupture, or distal embolization. When distal anastomotic pseudoaneurysms grow to more than 2 cm in diameter, graft interposition is recommended.
Graft limb thrombosis is a common reason for operative failure. Most patients are symptomatic. Management includes endovascular or surgical thrombectomy or extraanatomic bypass.44
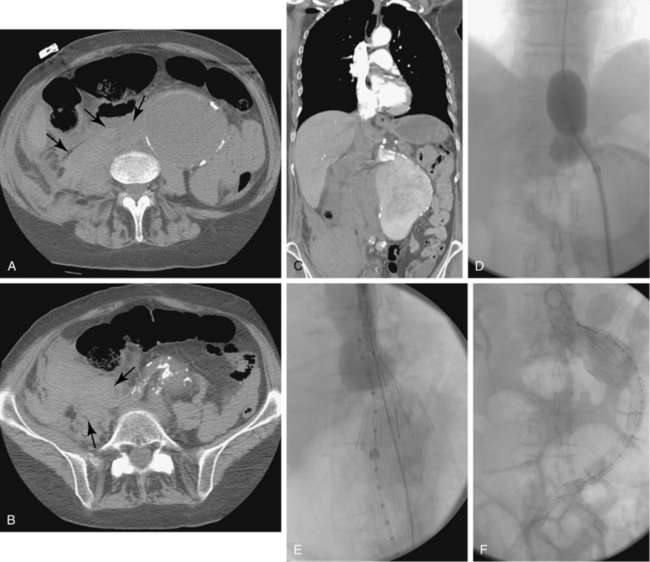
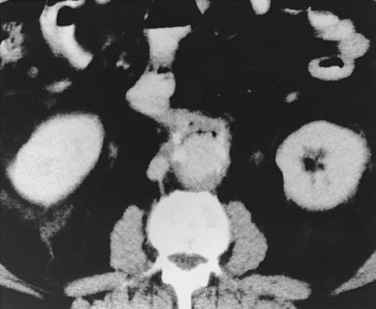
Aortoenteric fistula is observed in about 1% of cases of aortoiliac graft placement.65–67 The fistula between the bowel (usually duodenum) and the prosthetic material is almost always a result of graft infection. Most patients suffer gastrointestinal bleeding, fever, or both. A “herald” bleed may occur, presaging a massive hemorrhage. A few individuals display signs of graft infection but have no intestinal tract bleeding. CT is the standard imaging tool in suspected cases (Fig. 7-13). In addition to identifying perigraft fluid and gas in the graft, findings may include thickening of small bowel adjacent to the graft and exchange of contrast material between the small bowel and graft.
Endovascular Abdominal Aortic Aneurysm Repair
In 1991, Juan Parodi and colleagues68 reported the first endovascular placement of a homemade stent graft in a human for treatment of AAA. This seminal paper ushered in a period of intense investigation and rapid evolution of a remarkable alternative to the traditional operation. The anticipated advantages of endovascular repair over open operation (decreased blood loss and morbidity and mortality, shorter hospital stay, and speedier recovery) have largely been realized. However, the need for secondary procedures and lifelong surveillance and the risk (albeit small) of late rupture are still limitations of currently available techniques.
Preprocedure Imaging and Intervention
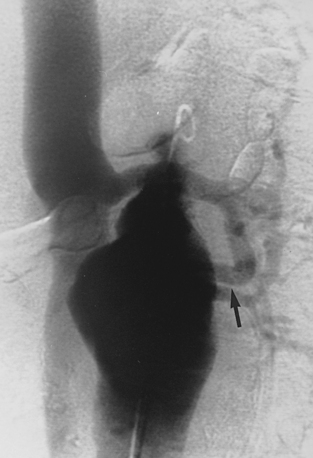
Patient and device selection and preprocedure planning demand high-quality noncontrast CT and CT angiography (with multiplanar reformatted images) followed by catheter angiography (including biplane aortography and pelvic arteriography in multiple projections)69,70 (Box 7-3 and Fig. 7-14).
Box 7-3 Criteria for Patient and Device Selection for EVAR
Abdominal Aorta
Common Iliac Arteries
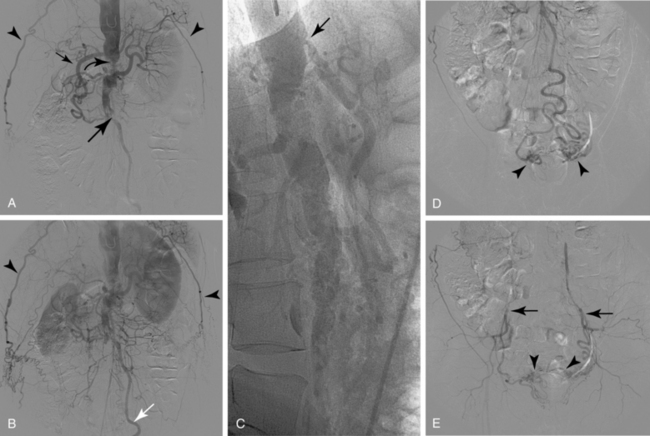
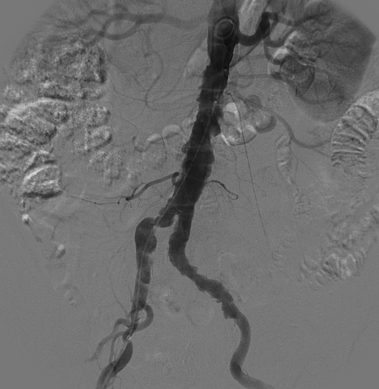
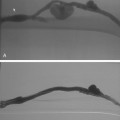
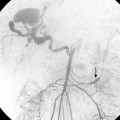
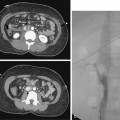
Sometimes, the endograft must be extended down into the external iliac artery because of concomitant aneurysm, extreme tortuosity, or short landing zone in the common iliac artery or the presence of an internal iliac artery aneurysm. In this situation (about 20% of cases), retrograde collateral flow through the ipsilateral internal iliac artery (from the contralateral vessel or lumbar or IMA collaterals) may continue to pressurize the aneurysm after the stent graft is placed. Internal iliac artery embolization is often performed before EVAR to avoid such endoleak (see later).71–76 Either simultaneous or staged bilateral occlusion is acceptable (Fig. 7-15). A cobra or long reverse curve catheter is directed into the main internal iliac artery from a contralateral approach. The embolic material of choice is stainless steel or platinum coils or the Amplatzer vascular occluder.75 An oversized coil is first deposited, followed by additional coils to create an occlusive “nest.”
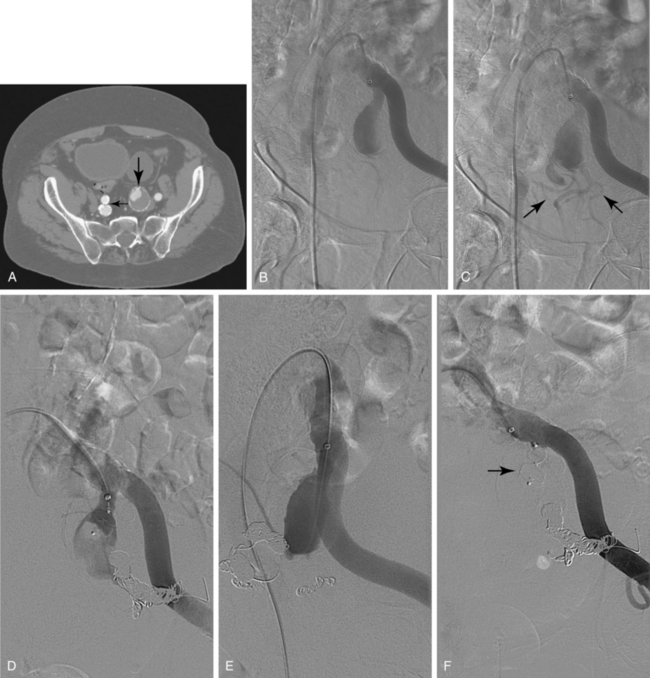
Figure 7-15 Internal iliac artery embolization prior to EVAR. A, Contrast-enhanced CT scan shows a large internal iliac artery aneurysm with extensive mural thrombus and intimal calcification (large arrow). Right internal iliac artery is dilated and calcified (small arrow).B and C, Catheter angiography outlines the aneurysm and branches beyond (arrows). These distal branches must be excluded first with multiple coils (D and E) to prevent backfilling of the aneurysm before more proximal embolization with an Amplatzer plug (F,arrow). Lesion is now completely excluded from the circulation.
This procedure, when performed properly, is highly effective in preventing subsequent endoleak related to the internal iliac artery. The downside is the risk for pelvic ischemia, which can present later as buttock claudication or impotence (about 25% of cases), bladder or colonic ischemia, or, rarely, nerve injury. These symptoms may be transient. The most important technical factor in preventing ischemic complications is occlusion of the main internal iliac artery trunk proximal to the bifurcation into anterior and posterior divisions.73,74,76,77