Gastrointestinal Stromal Tumors
Annick D. Van den Abbeele
Ertuk Mehmet
Richard J. Tetrault
In 1983, Mazur and Clark (1) described gastrointestinal stromal tumors (GISTs) as a distinctive subgroup of gastrointestinal mesenchymal tumors not classified as neurogenic or smooth muscle-derived. Kindblom et al. (2) hypothesized that these tumors may originate from the interstitial cell of Cajal in the normal myenteric plexus, an intestinal pacemaker cell. This hypothesis was confirmed by Hirota et al. (3) in 2000, who demonstrated that neoplastic GIST cells show ultrastructural features and express cell markers typical of the normal interstitial cell of Cajal (4). Today, on the basis of pathological features, most gastrointestinal mesenchymal tumors previously designated as smooth muscle tumors, such as leiomyomas, leiomyoblastomas, and leiomyosarcomas, are classified as GISTs (4).
GIST is the most common mesenchymal neoplasm of the alimentary tract (5). Although it is a rare tumor and accounts for only approximately 3% of all gastrointestinal cancers, up to 20% of small bowel malignancies are GISTs (6). GISTs predominantly occur in the fifth to seventh decades of life, without any gender predilection (4). Primary GIST is solitary rather than multiple. GISTs arise throughout the whole length of the gastrointestinal tract, most commonly in the stomach (60% to 70%) followed by small bowel (20% to 25%) and rarely in the rectum, esophagus, colon, and appendix (7). The presentation symptoms are usually nonspecific and are typically related to mass effects dependent on the size and the location of the tumor (4). Small GISTs are usually detected incidentally at surgery, on radiographic imaging, or during endoscopy and are typically asymptomatic. In approximately half of the patients, bleeding may be the first manifestation of GIST (4). Another 20% of patients will present with abdominal discomfort that is typically associated with a large tumor size. Another clinical presentation is bowel obstruction or perforation. At first diagnosis, approximately 10% of patients have metastatic disease (7), but this incidence may be higher, with DeMatteo et al. (8) reporting metastatic disease found in nearly half of their patients. The liver is the most common site (65%) for metastatic disease, followed by the peritoneum (21%), mesentery and abdominal cavity, while metastases to lymph nodes, bone, and lung are rare. Most patients develop recurrent GISTs after an apparently complete surgical resection of the primary lesion, with the most common sites of recurrence being in the liver, peritoneum, or both (9).
GIST has a typical immunohistochemical profile that helps confirm the diagnosis. Approximately 95% of GISTs are positive for KIT (CD117), the c-kit receptor tyrosine kinase (10). In most GISTs, an activating mutation of c-kit leads to ligand-independent receptor dimerization and activation of KIT tyrosine kinase that promotes tumor survival and tumor growth (11). More than 80% of GISTs have an oncogenic mutation in the KIT tyrosine kinase (12), and most of the mutations occur in the juxtamembrane domain encoded by exon 11, but mutations may also occur in exons 9, 13, 17, or PDGFRA (platelet-derived growth factor receptor) (13,14,15). Approximately 10% to 15% of GISTs are negative for KIT and PDGFRA mutations. Interestingly, the kinase genotype has been shown to be predictive of response to imatinib therapy (13,16). The presence, as well as the location of mutations in KIT or PDGFRA, highly correlates with the clinical response to imatinib, the first tyrosine kinase inhibitor approved for the treatment of GIST (see below) (13,16). Patients who have an exon 11 mutation within their tumors show the best response to imatinib therapy, with an 85% response rate. There is also some suggestion that patients with exon 9 mutations may be more sensitive to sunitinib (17), the second drug approved for treatment of GIST (see below). Recent data even suggest that mutational screening might be useful in selecting the optimal dose of imatinib (16). It is too early to determine if mutational screening will become part of a standard diagnostic work-up for patients with GISTs. However, it is interesting to see how far the knowledge about GIST, as well as the molecular-targeted therapies used to treat it, have come in a relatively short period since the first trial was started in the summer of 2000.
GISTs are known to be both chemoresistant and insensitive to irradiation, and surgical resection is the initial therapy for patients with primary GIST who have no metastases and are considered resectable. Prior to 2002, the lack of therapeutic options in inoperable and metastatic disease resulted in a generally poor prognosis in patients with GISTs (18,19,20). Recent studies, however, have shown promising results and greatly improved survival when patients with GISTs were treated with a new line of therapy using a selective small molecule that inhibits tumor growth by competitive interaction at the adenosine triphosphate–binding site of the c-kit receptor
(imatinib mesylate, STI571, Gleevec, Glivec; Novartis Pharmaceuticals, East Hanover, New Jersey).
(imatinib mesylate, STI571, Gleevec, Glivec; Novartis Pharmaceuticals, East Hanover, New Jersey).
Imatinib is an oral drug that inhibits several tyrosine kinases including KIT and BCR-ABL (20,21,22,23,24). The multidisciplinary team of Demetri et al. (20) participated in the first multicenter trial that tested imatinib in patients with unresectable and metastatic GISTs. The results of this trial showed a high incidence of durable responses with 85% of the patients still alive 76 weeks post-initiation of treatment. There was a close relationship between clinical outcome and the results seen on positron emission tomography (PET) using fluorine-18-fluoro-2-deoxy-D-glucose (FDG), or FDG PET, 1 month following initiation of imatinib, while objective response by computed tomography (CT) criteria (using the Southwest Oncologic Group [SWOG] criteria) were lagging weeks and months behind the response seen on FDG PET (25). Although no patient had achieved a complete response by standard anatomic criteria at that time point, clinical benefit was clearly observed even in patients with stable disease. It actually took a median of 3 months for patients to achieve partial response by SWOG criteria, while a marked decrease in glycolytic metabolism could be observed on FDG PET in all patients who responded to imatinib at 1 month, and even as early as 24 hours postinitiation of the drug (20,26).
Although most patients with advanced GISTs benefit from imatinib therapy, a small percentage of patients (less than 15%) may have primary resistance to the drug. Patients can also develop secondary resistance to the drug following a period of response to imatinib. This can occur months or years following continuous response to imatinib and is thought to be related to clonal differentiation within the tumor secondary to the acquisition of new mutations that confers resistance to the drug in this subpopulation of tumor cells (27).
In January 2006, the U.S. Food and Drug Administration approved a second line of molecular-targeted therapy (sunitinib malate, SU11428, Sutent; Pfizer, New York, New York) for the treatment of metastatic GISTs (28). This drug is an oral tyrosine kinase multitargeted inhibitor that inhibits KIT and platelet derived growth factor receptor (PDGFR), as well as vascular endothelial growth factor receptor (VEGFR)1-3, FLT-3, and RET, adding a potential antiangiogenic effect. It is available to patients who are unresectable and have developed primary or secondary resistance to imatinib and who are progressing despite higher doses of imatinib (28). Here again, very few patients achieved objective anatomic response criteria by RECIST (Response Evaluation Criteria in Solid Tumors) (29), but clinical benefit was observed in the 58% of patients who showed stable disease as the best overall tumor response by standard RECIST criteria. This discrepancy between clinical benefit and standard anatomic response criteria has now been observed with more than one tyrosine kinase inhibitor.
Gastrointestinal Stromal Tumors and Imaging Studies
Initial Evaluation
Although imaging studies play an important role in the management of patients with GISTs, there are no well-defined imaging examinations for the initial diagnosis of GISTs. Radiological investigations occasionally pick up incidental cases, but patients are typically referred for imaging to characterize an abdominal mass or to evaluate nonspecific abdominal symptoms, as discussed above, and CT and/or magnetic resonance imaging (MRI) are usually used in that context.
Ultrasonography, contrast-enhanced CT, and MRI are able to assess GISTs based on detailed morphological appearance, but CT is the modality of choice for characterizing and localizing an abdominal mass, as well as for the staging of biopsy-proven GISTs (4). Oral and intravenous contrast-enhanced cross-sectional imaging studies are strongly preferred over conventional planar radiography and barium studies in detecting and delineating both the primary disease and metastatic lesions.
CT may reliably localize GIST as well as determine tumor size and possibly reveal the presence of secondary metastatic localizations (e.g., hepatic metastases) (4). CT can also disclose an extraluminal mass originating from the digestive tract wall. The untreated tumor masses are usually dense and enhance following intravenous contrast administration. Large GISTs can be heterogeneous with areas of necrosis. Imaging features usually offer valuable information in distinguishing tumors of mesenchymal origin from lymphoma, where necrosis is less common, and from epithelial neoplasms of gastrointestinal tract.
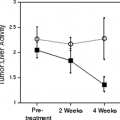
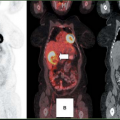
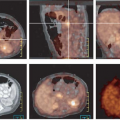
FDG PET is also particularly useful for the staging of GISTs because the great majority of untreated GISTs show intense glycolytic activity resulting in significant FDG uptake within all tumor masses (Figs. 8.20.1, 8.20.2A, 8.20.3A). However, FDG PET has mainly been used in the clinic for patient follow-up (4) (see below). Some GISTs have been reported to be FDG negative, but these tumors represent a small percentage of all GISTs. A baseline FDG PET should always be obtained prior to treatment if one is considering using this modality to monitor response to therapy.
FDG PET also allows for semi-quantitative evaluation of metabolic activity within the tumor using the standardized uptake value (SUV) or maximum SUV (SUVmax) to characterize the glycolytic metabolism of the tumor and to evaluate response to therapy. Metabolic response criteria have been defined by the European Organisation for Research and Treatment of Cancer (EORTC) (30). These criteria are based on the magnitude of the decrease in SUV relative to baseline (hence the importance of obtaining a baseline study), and provide definitions for complete, partial, progressive, and stable metabolic disease. The prognostic value of
FDG PET has also been shown in various settings and in a variety of cancers (31,32,33,34,35,36,37,38,39).
FDG PET also allows for semi-quantitative evaluation of metabolic activity within the tumor using the standardized uptake value (SUV) or maximum SUV (SUVmax) to characterize the glycolytic metabolism of the tumor and to evaluate response to therapy. Metabolic response criteria have been defined by the European Organisation for Research and Treatment of Cancer (EORTC) (30). These criteria are based on the magnitude of the decrease in SUV relative to baseline (hence the importance of obtaining a baseline study), and provide definitions for complete, partial, progressive, and stable metabolic disease. The prognostic value of
FDG PET has also been shown in various settings and in a variety of cancers (31,32,33,34,35,36,37,38,39).
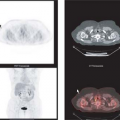
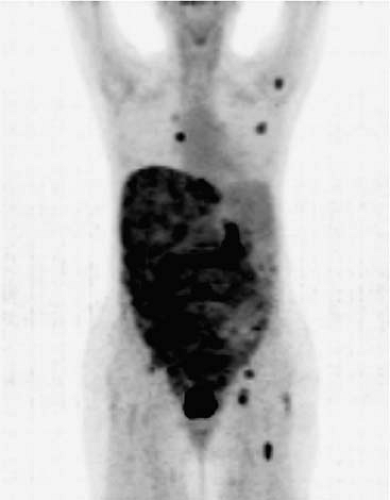 Figure 8.20.1. FDG PET in a patient with GIST with extensive metastatic involvement throughout the liver, the entire abdomen, and pelvis. The scan also demonstrates unsuspected skeletal/bone marrow metastases in the left humeral head, a right posterior rib, the pelvis, and the left femur, as well as unsuspected soft tissue involvement in the left breast and nodal involvement in the right internal mammary chain.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|