The use of a radionuclide to measure gastric transit was first described in 1966. Radionuclide gastric emptying scintigraphy has long been the standard methodology for measuring gastric transit. In this section, the recommended standardized gastric emptying protocol is described in some detail, followed by a discussion of esophageal and intestinal transit ( Fig. 10.1 ). Other topics include gastrointestinal bleeding, Meckel, peritoneal, and salivary gland scans.
Gastrointestinal Transit Scintigraphy
Gastric Emptying
The radionuclide gastric-emptying study is the accepted standard methodology used to measure gastric transit. It is physiological, quantitative, accurate, and reproducible. An upper gastrointestinal contrast study can detect a gross delay in gastric emptying, but it is not sensitive for the detection of less severe but symptomatic gastroparesis. Other methodologies have been proposed and investigated, most recently the wireless motility capsule; however, all have issues, and none has found routine clinical use. However, the radionuclide study cannot differentiate a severe functional delay from anatomical obstruction, such as a tumor or pyloric channel ulcer. Endoscopy or contrast barium radiography is required for that purpose.
Patients with delayed gastric emptying (gastroparesis) present clinically with symptoms of postprandial nausea, vomiting, and abdominal pain. Some of the clinical indications for the radionuclide gastric emptying study are listed ( Box 10.1 ). Rapid gastric emptying is less common than delayed. Patients may present with similar symptoms, although more commonly postprandial abdominal cramps, diarrhea, flushing, and tachycardia (dumping syndrome).
Insulin-dependent diabetics with persistent postprandial symptoms
Diabetics with poor blood glucose control
Nonulcer dyspepsia
Unexplained postprandial nausea, vomiting, and abdominal pain
Severe reflux esophagitis
To assess response to a motility drug
Anatomy and Physiology
The regions are designated from proximal to distal as cardia, fundus, body, antrum, and pylorus ( Fig. 10.2 ). Gastric mucosal glands secrete hydrochloric acid and digestive enzymes. Gastric motility is controlled by both neuromuscular gastric activity and small intestinal neuroendocrine feedback.
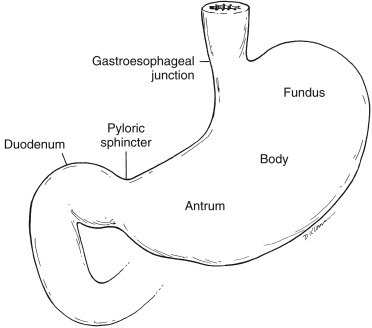
The gastric fundus and antrum have distinct functions. The more proximal fundus acts as a reservoir, accepting large meals with only a minimal increase in pressure (receptive relaxation and accommodation). Fundal tonic contractions produce a constant pressure gradient between the stomach and pylorus, resulting in liquid emptying. The more distal antrum has phasic contractions initiated by a neural pacemaker. Muscular contractions sweep down the antrum in a ring-like pattern, squeezing food toward the pylorus. Larger food particles are not allowed to pass and are retropelled. The solid material is converted into chyme through contact with acid and peptic enzymes and mechanical grinding. Food particles must be broken down until they are small enough to pass through the pyloric sphincter (1–2 mm). The antrum is responsible for solid emptying. The pylorus, at the junction of the antrum and duodenal bulb, acts as a sieve, regulating gastric outflow ( Fig. 10.3 ).
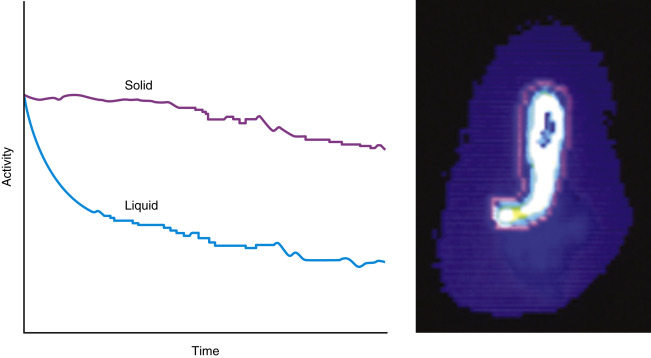
The pattern of emptying for solid and liquid meals is different. Solids have a delay before emptying begins (lag phase) lasting 5 to 25 minutes. This is the time required to grind the food into small enough particles so that they can pass through the pylorus. Following the lag phase, solids empty in a relatively linear pattern ( Fig. 10.4 ). The rate of emptying depends on the size and contents of the meal. Meals with greater volume, weight, carbohydrates, protein, or fat empty slower ( Box 10.2 ). Liquids have no delay before emptying begins. Clear liquids empty in a monoexponential pattern ( Fig. 10.5A and B ). Full liquids and clear liquids ingested simultaneously with a solid meal empty in a slower multiexponential pattern.
Meal content
Fat, protein, acid, osmolality
Volume
Weight
Caloric density
Particle size
Time of day
Patient position (standing, sitting, supine)
Gender
Metabolic state
Stress
Exercise
Gastric Stasis Syndromes
The majority of patients with chronic gastroparesis have a functional cause; that is, there is no known pathological etiology. An exception is diabetic gastroenteropathy, which occurs in patients with long-standing insulin-dependent diabetes. Here gastroparesis is caused by vagal nerve damage as part of a generalized autonomic neuropathy. In addition to producing disagreeable postprandial symptoms, the delay in emptying may exacerbate the problem of diabetic glucose control because the timing of the insulin dose with food ingestion and absorption may be unpredictable. A common gastric disorder is nonulcer dyspepsia, characterized by ulcer-like or dyspeptic symptoms. It is reported that gastroparesis occurs in 20% to 40% of these patients.
Most patients with gastroparesis have a chronic problem with delayed emptying. However, some have acute and potentially reversible causes (e.g., due to viral gastroenteritis, trauma, metabolic derangements). Common causes of and associations with chronic and acute gastric stasis syndromes are listed in Box 10.3 . Rapid gastric emptying is most commonly seen in patients who have had gastric surgery (e.g., pyloroplasty or gastrectomy), in patients with hyperthyroidism, or in those with a gastrinoma producing large amounts of the hormone gastrin (Zollinger–Ellison syndrome). A minority of patients with insulin-dependent diabetes have rapid gastric emptying ( Box 10.4 ), with symptoms of palpitations, diaphoresis, weakness, and diarrhea.
Acute Gastroparesis—Potentially Reversible
Trauma
Postoperative ileus
Acute viral infections (e.g., gastroenteritis)
Hyperalimentation
Metabolic: Hyperglycemia, acidosis, uremia, hypokalemia, hypercalcemia,
hepatic coma, myxedema
Physiological: Labyrinth stimulation, physical and mental stress, gastric distention, increased intragastric pressure
Hormone increases: Gastrin, secretin, glucagon, cholecystokinin, somatostatin, estrogen, progesterone
Chronic Gastroparesis
Anatomical
Gastric ulcer
Surgery, vagotomy
Pyloric hypertrophy
Postradiotherapy
Tumors
Diabetic gastroenteropathy
Functional
Nonulcer dyspepsia
Dermatomyositis
Systemic lupus erythematosus
Amyloidosis
Hypothyroidism
Familial dysautonomia
Pernicious anemia
Tumor-associated gastroparesis
Progressive systemic sclerosis
Fabry disease
Prior surgery
Pyloroplasty
Hemigastrectomy (Billroth I, II)
Diseases
Duodenal ulcer
Gastrinoma (Zollinger–Ellison syndrome)
Hyperthyroidism
Diabetics (subgroup)
Hormones
Thyroxine
Motilin
Enterogastrone
Patient Preparation
Hyperglycemia per se, independent of diabetic gastroenteropathy, may cause a delay in gastric emptying. Thus, gastric-emptying studies should be performed when patients are under good diabetic control, with a fasting blood preferably <250 mg/dL. Also, many commonly used nongastric therapeutic drugs can delay gastric emptying ( Table 10.1 ). In our clinic, we allow the referring physician to decide whether the patient should continue to take his or her medications before the study. This will depend on the study indication (e.g., whether to make the diagnosis of gastroparesis or to determine whether a specific therapeutic drug has been effective). When indicated, the drugs should be stopped 48 to 72 hours before the study. Medications taken should be considered when interpreting the study.
| Drug Type | Specific Drugs |
|---|---|
| Cardiovascular | Calcium channel blockers (e.g., nifedipine) Beta-adrenergic antagonists (e.g., propanolol) |
| Respiratory | Isoproterenol, theophylline |
| Gastrointestinal | Sucralfate, anticholinergics, tegaserod |
| Reproductive | Progesterone, oral contraceptives |
| Neuropsychiatric | Valium, Librium, Librax, Ativan, tricyclic antidepressants, levodopa Phenothiazines (e.g., Thorazine) |
| Opiates Alcohol and nicotine | OxyContin, Percodan, Percocet |
Therapy of Gastroparesis
Metoclopramide (Reglan) is the most common therapeutic drug prescribed for delayed gastric emptying. It is not effective in all patients, symptom relief is not always accompanied by an improvement in gastric emptying, and serious side effects may occur in some patients (e.g., tardive dyskinesia seen in 10% of patients taking the drug for longer than 3 months). Domperidone (Motilium), used in Europe, is not approved for clinical use in the United States. Cisapride (Propulsid) was removed from the U.S. market due to serious arrhythmias. Erythromycin, a motilin agonist, improves gastric motility; however, it has a high incidence of nausea and vomiting. Analogs of erythromycin as well as other potential therapeutic drugs are in development. Surgical intervention is sometimes used to treat refractory gastroparesis. One approach is implantation of an electrical gastric stimulator. In patients who are not responsive to standard therapies, gastric resection may be indicated.
Radiopharmaceuticals
For accurate and reproducible quantification of solid gastric emptying, the radionuclide must be tightly bound to the solid component of the meal. Elution of the radiolabel results in a part-solid, part-liquid labeled mixture and may be quantitatively unreliable. Radiolabeled eggs are most commonly used for solid gastric-emptying studies. Tc-99m sulfur colloid (Tc-99m SC) binds to albumen in egg white during cooking. Although whole eggs work well for gastric emptying, egg whites have higher percent binding. Tc-99m SC remains stable in the acidic stomach and is not absorbed by the gastrointestinal (GI) tract.
For liquid meals, the radiotracer must equilibrate rapidly within the liquid and be nonabsorbable in the gastrointestinal tract. Tc-99m- or In-111-labeled diethylenetriaminepentaacetic acid (DTPA) and Tc-99m SC meet these criteria. Dual-phase solid-liquid studies use one radiotracer for the solid meal and another for the liquid phase, for example, In-111 DTPA as the liquid marker (171, 247 keV) and Tc-99m SC (140 keV) as the solid marker ( Fig. 10.6 ). They are differentiated by their separate photopeaks.
Standardization of Solid Gastric-Emptying Scintigraphy
Various methodologies have been used over the years for solid gastric-emptying studies, including different meals, patient positioning, instrumentation, framing rate, and study length as well as quantitative methods. All of these factors may affect normal values. Thus, normal values must be well validated for the specific meal and methodology used. In the past, gastroenterologists have expressed concern about the different methodologies and different normal values. They advocated for a standardized gastric emptying protocol.
Standardized Solid Gastric-Emptying Study
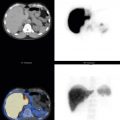
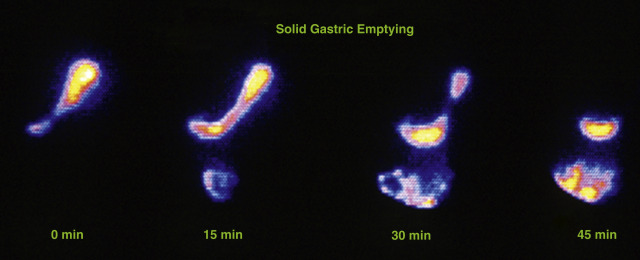
In 2008, an expert panel of gastroenterologists and nuclear medicine physicians published Consensus Recommendations for Radionuclide Gastric Emptying. The recommendations were based on a protocol published by Tougas and colleagues in 2000 and are described in detail in Box 10.5 . The protocol was selected because it simplified the procedure and because the normal values were considered valid based on the large number of normal subjects studied (123). The meal consists of an egg-substitute sandwich (4 oz egg white, equivalent to 2 whole eggs, 2 slices of bread), strawberry jam (30 g), and water (120 mL). The study requires 1-minute anterior and posterior images acquired at four time points (immediately after meal ingestion and at 1, 2, and 4 hours) and calculation of the percent gastric retention at each time point ( Figs. 10.7–10.9 ).
Patient Preparation
The referring physician should decide which medications are to be continued/discontinued before the study.
For diabetics, a fasting blood sugar (FBS) is recommended before the study. The study should not be performed if the FBS is >250 mg/dL.
Begin the study in the morning after an overnight fast. The patient should be NPO for at least 6 hours before study.
Radiopharmaceutical and Meal
Tc-99m SC 1 mCi (37 MBq) bound to 4 oz egg substitute (Egg-Beaters or generic egg-white equivalent) by microwaving or scrambling after mixing egg with Tc-99m SC. Stir once or twice while cooking until mixture reaches consistency of omelet. Meal also consists of two slices of white bread, strawberry jam (30 g), and water (120 mL).
Meal should be ingested within 10 to 15 minutes.
Instrumentation
Gamma camera: Large-field-of-view, dual-detector camera
Collimator: Low energy, parallel hole, high resolution or general purpose
Tc-99m photopeak with a 20% window around 140 keV photopeak
Computer setup: 1-minute frames (128 × 128 word mode matrix)
Patient Position
Position patient either standing upright or lying supine, with camera heads anterior and posterior. A single-head camera can be used, acquiring an image first anteriorly, then posteriorly.
Imaging Procedure
Acquire 1-minute frames at time 0 (immediately after ingestion) and 1, 2, and 4 hours; 3-hour images are optional.
Processing
Draw region of interest for anterior and posterior stomach views.
The geometrical mean of the anterior and posterior views is determined at each time point. The percent retention or gastric emptying is calculated at each imaging time point.
Decay correction is mandatory.
Interpretation
Delayed gastric emptying:
1 hour <10% emptying (>90% retention)
2 hours <40% emptying (>60% retention)
4 hours <90% emptying (>10% retention)
Rapid gastric emptying:
>70% emptying at 1 hour
>90% emptying at 2 hours
These values apply to the entire meal and are not valid for different meals or incomplete ingestion of the standard meal.
NPO, Nil per os; SC, sulfur colloid.
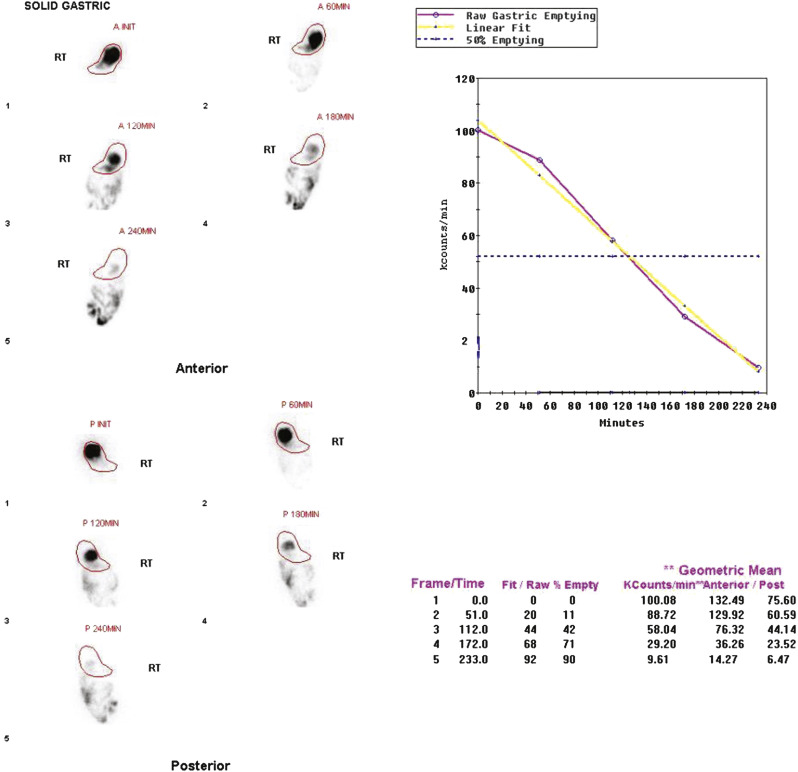
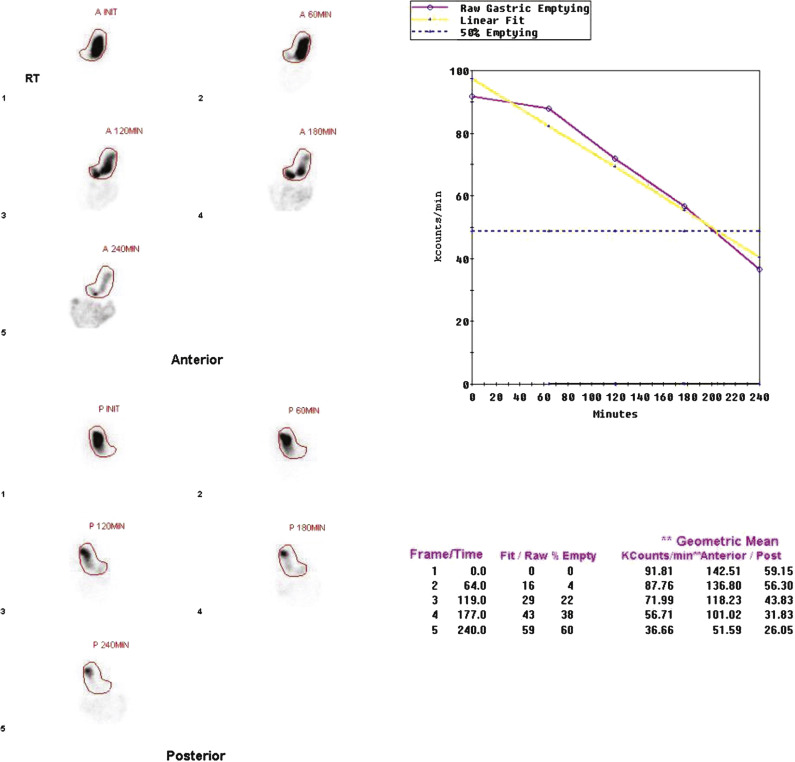
The Tougas and colleagues publication found that infrequent imaging (each hour) was as accurate as more frequent imaging (e.g., every 10 minutes). The rationale for a 4-hour study length was based on several publications reporting a higher rate of detection of gastroparesis at 4 hours compared with 2 hours. One investigation reported that by extending the study from 2 to 4 hours, the number of patients diagnosed with gastroparesis increased by 30%. Widespread use of this protocol ensures that results are comparable between institutions. Although a 4-hour study length might at first seem demanding of clinic logistics, the standardized protocol can actually improve patient flow. Multiple patients can be studied on one camera in a single morning because total imaging time per patient is short.
Interpretation of Solid Gastric-Emptying Studies
A standardized gastric-emptying study is considered normal if the 2-hour retention value is <60% retention (>40% emptying) and the 4-hour value is <10% retention (>90% emptying; see Fig. 10.7 ). The Tougas protocol used “percent retention” for the normal values. This textbook prefers “gastric emptying.” Normal values are based on ingestion of the entire meal. Ingestion of a smaller volume or less eggs, bread, jam, or water will likely result in more rapid emptying than if the entire meal was ingested. Normal values have not been published for partial meal ingestion. Thus, if a patient cannot ingest the whole meal, a statement should be added to the interpretation (e.g., “Because the patient did not ingest the entire meal, the study likely overestimates the rate of gastric emptying”).
Delayed emptying may occur at both 2 and 4 hours, only at 2 hours, or only at 4 hours. All these patterns are abnormal and may be causing the patient’s symptoms. The study can be discontinued early (e.g., 2 hours) if the 4-hour normal values have been achieved. Some clinics obtain a 3-hour image for this same reason, potentially stopping the study early if there has been >90% emptying. Rapid emptying has been defined as >70% emptying at 1 hour (see Fig. 10.9 ).
Two published large investigations have now reported that the solid gastric-emptying study can be stopped at 2 hours in >50% of patients because the 2-hour emptying value can predict 4-hour emptying in those patients. If 2-hour gastric emptying is <35%, the study can be interpreted as delayed emptying and stopped. If there is >55% emptying, it can be interpreted as normal with high accuracy and the study discontinued. If emptying is between those two values, the study must be continued to 4 hours. The disadvantage of stopping early is that it may be more difficult to compare subsequent follow-up studies.
Alternative Solid Meals
Some patients cannot eat eggs for dietary or allergic reasons. Limited data exist regarding normal values for alternative meals. One small published study found that EnsurePlus (8 oz) had normal values that did not differ significantly from the standardized meal, in spite of the fact that it is a full liquid meal. A few other meals have been reported with established normal values, but their contents and use are often unique to their country of origin. One alternative meal with well-established published normal values is a clear liquid-meal, described in the following section. It can be used when the patient cannot ingest the standard meal or EnsurePlus.
Liquid Gastric Emptying
Accepted teaching had long been that liquid gastric-emptying studies were less sensitive for the detection of gastroparesis than solid studies, that liquids become abnormal only in late stages of gastroparesis, and that only a solid gastric-emptying study is necessary for clinical purposes. However, no data had been published to support this, and it has recently proven to be incorrect.
Two publications of 140 patients have compared clear-liquid (water)-only emptying with the standardized solid gastric-emptying meal in patients referred for suspected gastroparesis. More patients had abnormal liquid than solid emptying. The most important finding was that 25% of patients who had a normal solid study had delayed liquid emptying. The pathophysiologic explanation for these findings is unclear; however, it may help explain the patient’s symptoms. Many of our patients are referred for both studies.
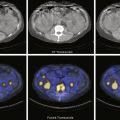
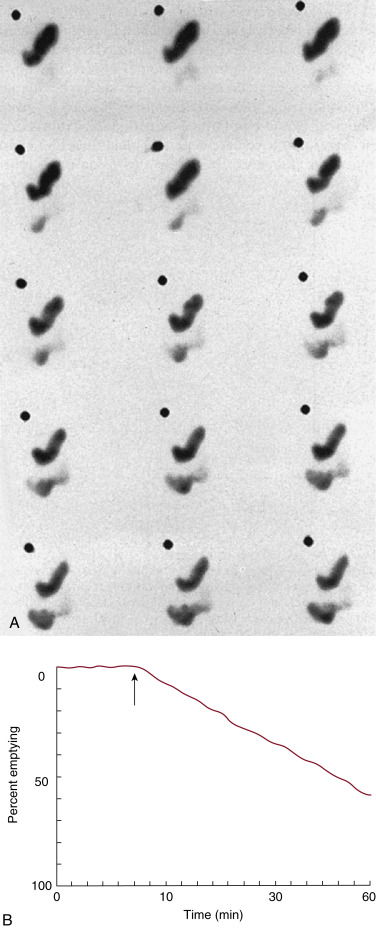
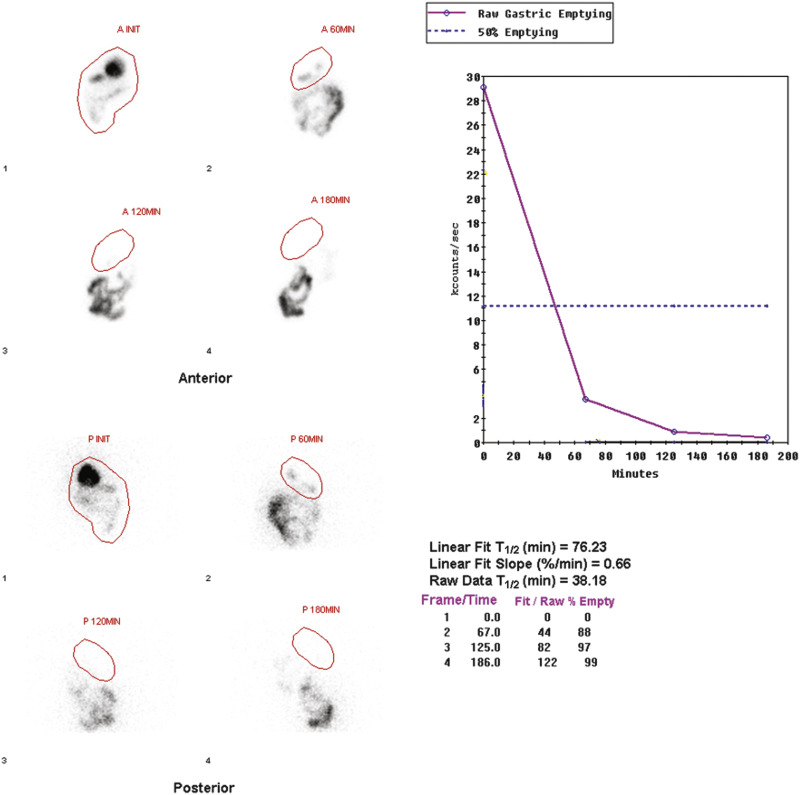
The methodology for clear-liquid-only studies is detailed in Box 10.6 . Because water empties rapidly, only a 30-minute study is required (1-minute framing rate). Imaging begins immediately after ingestion of the 300 mL water containing 1 mCi Tc-99m DTPA or Tc-99m SC. Alternatively, the liquid and solid studies can be performed sequentially on the same day, the clear liquid first with In-111 DTPA, followed by the solid study labeled with Tc-99m SC. Simultaneous evaluation of liquid and solid gastric emptying is possible and done at some institutions using a dual-isotope acquisition technique ( Fig. 10.10 ). Liquids radiolabeled with In-111 and ingested simultaneously with the standardized Tc-99m-labeled solid meal empty slower than liquid-only meals. The results of liquid-only and simultaneously ingested liquids and solids correlate poorly and likely provide different pathophysiologic information, although at present the reason is uncertain. However, if abnormal, either may explain the patient’s symptoms.
Patient Preparation
Patient must fast for 4 to 6 hours before study.
Radiopharmaceutical and Meal
Tc-99m DTPA or Tc-99m SC, 1 mCi (37 MBq) in 300 mL water
Instrumentation
Gamma camera: Large field of view, dual detector
Collimator: Low energy, parallel hole, high resolution or general purpose
Tc-99m photopeak with a 20% window around 140 keV
Computer setup: 1-minute frames (128 × 128 word mode matrix) × 30
Patient Position
Semiupright (30–45 degrees) on a hospital gurney with the gamma camera placed in the left anterior oblique (LAO) projection (makes it convenient for patient to ingest meal and imaging be started promptly with the camera in the LAO projection)
Imaging Procedure
Have patient swallow radiolabeled water. Immediately after ingestion of the water, acquire 1-minute images for 30 minutes.
Processing
Draw region of interest (ROI) to outline stomach on all images. Correct for decay.
Observe for ROI overlap of the stomach with small intestines. If there is overlap, redraw ROI to exclude overlap.
Calculate a half-time of emptying and exponential T ½ .
Interpretation
Normal values: Less than 25 minutes
Use the half-time of emptying and/or exponential fit depending on which best represents the apparent emptying.
DTPA, diethylenetriaminepentaacetic acid; NPO, nil per os; SC, sulfur colloid.
Quantification of Gastric Emptying
Various methods have been used over the years to quantify gastric-emptying studies. However, investigations have found no clinical advantage to more frequent imaging, other methods of quantification, or calculation of the lag phase. Percent emptying is more accurate than a rate of emptying (T ½ ) with the standardized methodology because there are only 4 to 5 data points. For the standardized solid gastric-emptying study, a gastric region of interest (ROI) is drawn for all anterior and posterior views at each time point (see Figs. 10.7–10.9 ). Decay and attenuation correction is required for accurate quantification. The percent emptying is calculated at each time point.
Percent emptying = initial meal counts minus meal counts at 1, 2, and 4 hours, each divided by initial meal counts, and all corrected for attenuation and decay.
Attenuation Correction
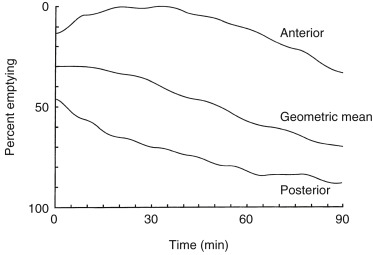
Attenuation on gastric-emptying studies varies as the ingested meal transits the stomach because stomach contents move from the relatively posterior fundus to the more anterior antrum. With a single gamma camera detector positioned anteriorly, the counts increase during the early portion of the study in spite of the fact that all the food is already in the stomach from time zero. This results in a quantitative error. Attenuation correction is mandatory for correct results (see Fig. 10.10 ). Otherwise, there may be an underestimation of emptying, by as much as 10% to 30%. This is most pronounced in obese patients; however, it also occurs in nonobese patients and is not predictable between patients. The commonly used method for attenuation correction is mathematical and involves calculating the geometrical mean (square result of the product of the anterior and posterior counts) at each time point.
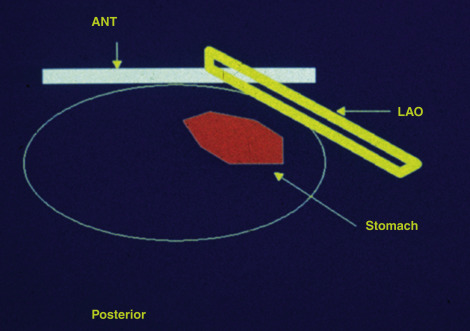
The left anterior oblique projection acquisition method is an alternative approach that minimizes the effect of attenuation. The study is acquired in this one projection. It compensates for attenuation because the head of the gamma camera is roughly parallel to the movement of the stomach contents, and thus the effect of attenuation is minimized ( Fig. 10.11 ). The advantages of this method are that it requires only a single-headed camera, and no mathematical correction is needed. Results correlate well with those of the geometrical mean method, although the geometrical mean is more accurate and preferable whenever possible ( Fig. 10.12 ).

Correction for radioactive decay is mandatory for solid-meal studies because of the short half-life of Tc-99m (6 hours) and the relatively long duration of the study (4 hours). Down-scatter (In-111 into the Tc-99m window) and up-scatter (Tc-99m into the In-111 171 keV window) have been shown to be inconsequential if the administered dose ratio of Tc-99m to In-111 is at least 4:1. Therefore, with standard doses of 1 mCi Tc-99m and 100 to 200 μCi In-111, no correction is necessary.
Liquid Gastric Emptying Quantification
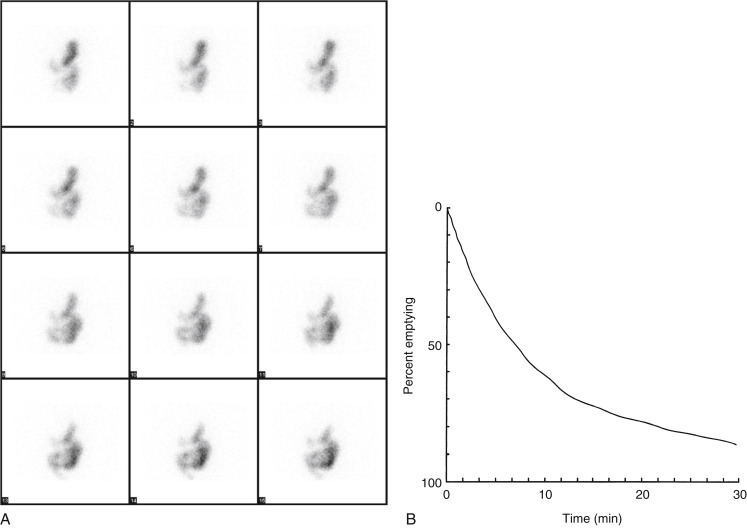
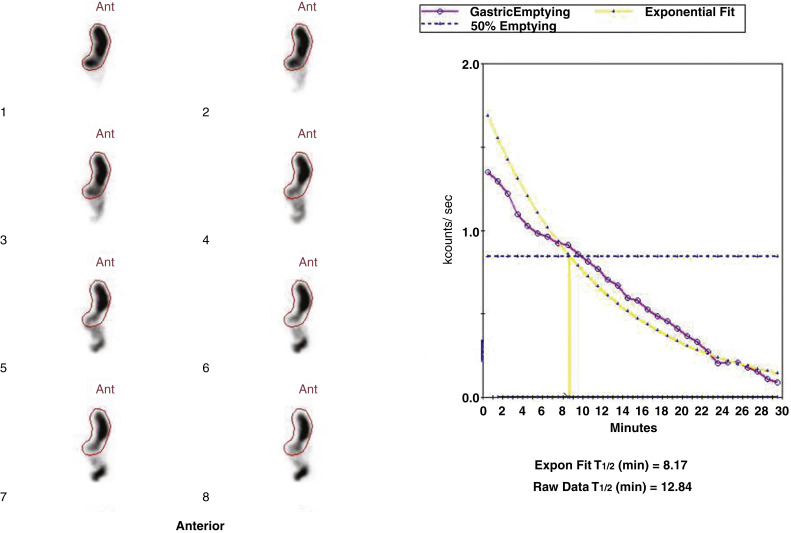
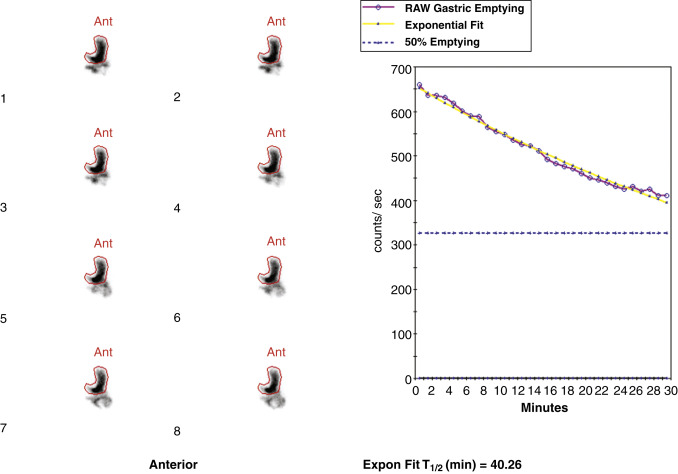
Attenuation correction is not necessary for liquid gastric emptying because of its rapid transit. A gastric ROI is drawn, and a half-time of emptying (time in minutes when counts become half of peak counts) and/or an exponential mathematical fit T ½ is calculated ( Figs. 10.13 and 10.14 ). If small bowel activity moves into the ROI, it should be excluded to correct for potential artificial delay. Normal values for a clear-liquid (water) study alone have been well validated, with a half-emptying time of <25 minutes. Adding salt, sugar, and so forth to the clear liquid meal slows the rate of emptying.
Gastric Accommodation
Some patients with symptoms suggestive of gastroparesis are found to have normal emptying. Some of these patients have a problem with gastric accommodation, the ability of the gastric fundus to relax with ingestion of a meal. Direct methods are insufficient and unpleasant for clinical diagnosis. The radionuclide approach is straightforward and simple for the patient. Tc-99m pertechnetate is injected intravenously. Single-photon emission computed tomography (SPECT) of the stomach is acquired. The patient ingests a meal, and then a second SPECT is obtained. The stomach volume is calculated before and after the meal. When ratios are compared with normal values, a diagnosis can be made. This has been a diagnostic test at only a limited number of centers around the world, but interest is increasing at other centers.
Esophageal Transit Scintigraphy
Abnormal esophageal transit usually presents with dysphagia or chest pain and occurs in patients with achalasia, scleroderma, systemic lupus, muscular dystrophy, polymyositis, diffuse esophageal spasm, and so forth. Barium swallow esophagrams can detect mucosal changes and anatomical lesions but provide only a rough qualitative assessment of motility. Manometry measures esophageal pressure, peristalsis, and sphincter contraction/relaxation but not transit ( Fig. 10.15 ). Esophageal transit scintigraphy is a time-proven test with good accuracy, particularly for achalasia and scleroderma. It is most commonly ordered today to screen patients who have a low suspicion of a motility disorder, to avoid invasive manometry, or to evaluate patient response to therapy. Sensitivity is reported to be 92% and specificity 88% for abnormal transit. However, it is not particularly useful in differentiating the underlying cause.
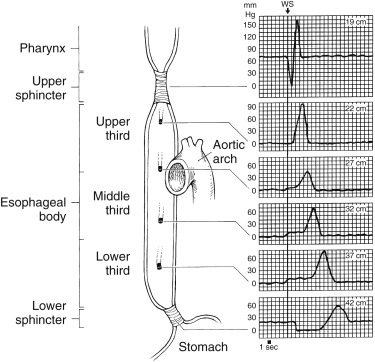
Methodology for Esophageal Transit Scintigraphy
Tc-99m SC or Tc-99m DTPA, 100 to 250 μCi, in 5 mL water is swallowed as a bolus. A clear-liquid protocol is described ( Box 10.7 ). Acquisition in the posterior view allows for easier administration of the liquid bolus and closer observation of the patient. The supine position eliminates the effect of gravity on esophageal clearance and thus is thought to be more sensitive for detection. However, in achalasia, gravity is the only emptying mechanism, and thus upright positioning is required for serial studies. Multiple swallows are suggested for complete esophageal emptying and to increase the sensitivity for abnormal transit in mild disease. Limited data suggest that semisolid meals may be more sensitive for detecting esophageal dysmotility; however, data are limited.
Patient Preparation
Patient should fast overnight.
Place radioactive marker next to cricoid cartilage.
Position the camera posteriorly.
Patient should be standing or supine (must be upright for achalasia).
Practice swallow(s) with nonradioactive bolus.
Radiopharmaceutical
Tc-99m SC or DTPA, 100 to 250 μCi (11 MBq) in 10 mL of water
Instrumentation
Camera setup: Tc-99m 140 keV photopeak with 20% window
Computer setup: 0.5-second frame × 120; byte mode, 64 × 64
Swallowing Procedure
Practice swallow with nonlabeled bolus.
Swallow radiolabeled liquid as a bolus.
Processing and Quantification
Time–activity curves, condensed dynamic images
Calculate
Transit time:
Time of initial esophageal entry until all but 10% of peak clears (abnormal >15 sec)
Percent emptying:
Maximal counts—counts 10 seconds after maximal counts divided by maximal counts before swallow (normal >83%)
DTPA, diethylenetriaminepentaacetic acid; SC, sulfur colloid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree