8
General Principles of Embolization and Chemoembolization
Transcatheter embolization is a frequently performed procedure that can be used for myriad clinical indications. Whether used to stop active bleeding, as palliative therapy for benign or malignant lesions, as a form of organ ablation, or in combination with chemotherapy as primary oradjunctive therapy for malignancies, embolization procedures are becoming more common in both large medical centers and community practice settings.
This chapter outlines general principles of embolic and chemoembolic therapy, with particular attention to the techniques used and to which clinical entities may benefit from such catheter directed therapy.
 Technique
Technique
Delivery systems
Depending on the size of the vessel or vessels to be embolized and the embolic agent to be used the two general types of delivery methods are selective catheter and coaxial catheter systems. Selective catheterization usually is performed with preformed curved catheters ranging from 5 to 7 Fr in diameter. Techniques used in selective catheterization are discussed in other chapters; most commercially available tapered end-hole catheters are sufficient for embolization procedures. The advent of coaxial systems has allowed superselective catheterization with minimal risk to the selected vessel. Many systems, such as the Tracker-325 system (Target Therapeutics, San Jose, CA), consist of a 3 Fr catheter with a large inner lumen diameter that allows delivery of sizable embolic materials such as Gelfoam pledgets (Upjohn, Kalamazoo, MI), or microcoils, which do not appreciably risk occluding the catheter. By using a coaxial system, the operator may catheterize the vessel of choice virtually without regard to vessel size, thus significantly decreasing the risk of both nontarget embolization and damage to the underlying vessel.
Balloon-occlusion catheters (BOCs) are useful for embolization procedures, particularly when alcohol is used as the embolic agent (see later). BOCs are compliant balloons designed to conform to the shape of the vessel in which they are inflated, in contrast to angioplasty balloons, which are designed to produce a significant radial force intended to fracture a plaque. Angioplasty balloons should not be used as BOCs, because of the notable potential risk of dissection of the vessel in which the balloon is inflated.
When large particles such as Gelfoam pledgets are used for embolization, care must be taken to use a selective catheter or coaxial system with an inner diameter and taper large enough to prevent occlusion of the catheter by the embolic material. A catheter with an end hle only also should be used to prevent inadvertent nontarget embolization through a catheter side hole. In addition, if an adhesive material such as cyanoacrylate is used, the glue must be mixed with tantalum powder for radiopacity and the delivery catheter must be flushed with dextrose solution, because exposure of the glue to ionic compounds causes instant polymerization and catheter occlusion.
Embolic agents
The number of embolic agents has increased significantly recently; however, it must be kept in mind that a combined approach using multiple agents is often most useful.
To choose the embolic agent needed, the anticipated end result of the embolization procedure must be understood. For instance, embolization for bleeding from a posttraumatic pseudoaneurysm has a different goal from embolization/ablation of a renal tumor preoperatively. Similarly, embolization for bleeding from a renal tumor has a purpose different from that of preoperative embolization of the same pathology. Large embolic agents such as coils and Gelfoam pledgets may be used for embolization of large vessels in organs with abundant collateral supply, such as the stomach or liver, in which proximal vessels can be occluded safely without risk of tissue infarction due to the extenssive collateral network. Large agents may be also used in tissues with an end-organ vascular supply in which the entire blood supply to the area of interest is to be occluded; however, large agents should not be used in vessels in which future embolization or transcatheter therapy is anticipated. By occlusion of the only vascular supply to a region, future selective catherization will be precluded by occluding the sole vascular access to the pathology. In contrast to large agents, liquid agents such as alcohol or glue will embolize to the most distal vascular supply and cause infarction by occluding the smallest arterioles supplying a lesion. Because collateral supply occurs at a site proximal to the embolization, liquid embolization is effective in destroying tissue even when there is significant parasitization or collaterization of blood supply from an adjacent source; however, it should not be used unless cell death and necrosis are the desired end results.
A second characteristics that must be considered when choosing an embolic agent is the desired degree of permanence of the occlusion. In particular, some materials are biodegradable, and recanalization of the embolized vessel should be anticipated. Most vessels embolized with Gelfoam pledgets and powder will recanalize within 2 to 3 weeks.1 Starch microspheres represent another biodegradable agent with an in vivo half-life of 20 to 30 min.2 Angiography 1 week after cross-linked collagen embolization of the hepatic artery reveals a normal hepatic arterial system and more complete return within 3 months.3 Autologous blood clots, a fourth degradable agent, that are currently used infrequently because of the ready availability of other agents.
Polyvinyl alcohol (PVA) particles are a commonly used permanent agent, with sizes ranging from approximately 50 to 2800μm. Embolization with PVA particles usually causes a permanent occlusion of vessels that corresponds to the size of the particles used. Other materials that have been used experimentally or in clinical trials include polylactic acid microspheres,4 ethylene vinylacetate copolymer particles dissolved in polyvinyl alcohol,5 and human dura mater.6
Embolization with particulate agents is considered complete when slowing of flow, not complete statis, is visualized. Because all arteries will tend to “back thrombose” to the previous bifurcation point when a distal occlusion is encountered, excessive embolization will decrease outflow enough that the entire feeding artery may thrombose. Fluoroscopically noted reflux is another indicator that the embolization procedure should be stopped.
 Use of embolization in Clinical settings
Use of embolization in Clinical settings
Nonneoplastic
Active bleeding
Hemorrhage is a clinical setting in which transcatheter embolotherapy may prove useful. Embolization of active bleeding almost always provides a less invasive form of patient management than surgical alternatives, and in certain clinical settings, such as postradiation or postsurgical fields, it may prove to be the only viable alternative because of the difficulty of surgery in such an operative field. Active bleeding may be caused by a number of etiologies, including inflammation, neoplasm, trauma, and congenital or developmental abnormalities. Once again, the desired end result of the embolization and the degree of permanence required are essential factors in determining the embolic agent of choice.
Because bleeding caused by inflammation is usually a temporary situation (i.e., over time and with appropriate medical management, the artery will likely stop bleeding), a temporary embolic occlusion is usually sufficient. In such cases, embolotherapy is a temporizing procedure performed to allow coagulation or healing tooccur within the vessel. In addition, because inflammatory etiologies may cause further bleeding within the same organ later, a temporary occlusion is desired so that future embolizations may be performed if needed. Finally, because the desired end result is not organ ablation or cell death but rather cessation of bleeding and tissue preservation, distal embolic agents, such as liquid or small particles, are usually contraindicated in inflammatory causes of hemorrhage.
Two inflammatory etiologies in which embolotherapy has proven efficacious include gastrointestinal (GI) and pulmonary hemorrhage. Transcatheter embolization of GI bleeding is documented best in the setting of benign causes, such as peptic ulcer disease, erosive gastritis, and Mallory-Weiss tears. In the case of peptic ulcer disease, most patients undergo endoscopy with an attempt at sclerotherapy; however, in patients in whom sclerotherapy fails or with recurrent bleeding after initial medical and endoscopic therapy, patients may be referred for angiography and possible embolotherapy. In addition, unstable patients who have massive upper GI bleeding may benefit from emergent embolotherapy preceding, or in lieu of, surgical therapies.7 Embolic agents used in the treatment of peptic ulcer bleeding have included Gelfoam pledgets, stainless steel coils, PVA particles, glue, and autologous clots.7–9 Occlusion of the more distal vasculature with agents such as glue prevents the formation peripheral collateral blood supply, thereby predisposing the embolized tissue to infection and increasing the risk of postembolization ischemic complications such as fibrosis and duodenal stenosis.8 Central occlusion with larger agents, such as coil occlusion of the gastroduodenal artery, allows collateral vessels to supply the distal tissue and precludes tissue infarction without compromising success of the embolization procedure.8 Gelfoam pledgets are the most commonly used agent; however, because temporary occlusion may or may not be sufficient, permanent central occlusion with coils is another possible therapeutic alternative.7–9 In the setting of massive upper GI bleeding without angiographic evidence of a bleeding site, embolization of the left gastric artery with Gelfoam pledgets or coils decrease the risk of recurrent GI bleeding (0% versus 50%).10 Embolization of the left gastric artery without angiographic documentation of bleeding can be justified because approximately 85% of gastric hemorrhage is caused by a lesion supplied by the left gastric artery.11
Embolotherapy for GI bleeding from benign causes other than peptic ulcer disease also has been successful. One study demonstrated technical success in all patients treated with emergent Gelfoam pledget and coil embolotherapy for active small intestinal hemorrhage; five of six (83%) patients survived hospitalization.9 Similar success rates have been noted in other studies for pathology as varied as jejunal ulcers, pseudoaneurysms, and Meckel’s diverticulum.12
Transcatheter embolotherapy in the treatment of hemoptysis also has proved efficacious.13,14 Because of the dual vascular supply to the lungs, the likelihood of postembolization tissue infarction is low, even with use of distal embolic agents such as PVA particles or liquid agents. Most pulmonary hemorrhages resulting from benign causes arise from the bronchial circulation; embolotherapy, therefore, typically is performed through the systemic circulation rather than the pulmonary arteries. There is great variability in the number and origins of the bronchial arteries; 43% of patients demonstrate a common trunk supplying the left and right bronchial arteries, and most patients demonstrate two or three bronchial arteries in total.15 Although they are small in the normal setting, the bronchial arteries become significantly enlarged in the setting of chronic inflammatory disease. Concurrent with bronchial artery enlargement, systemic-to-pulmonary artery communications develop, exposing the pulmonary arterial bed to greatly elevated pressures and causing rupture of the pulmonary arteries into contiguous airways.16 It is therefore from these pulmonary arteries that bleeding arises; however, pressure in the pulmonary arteries normalizes after the bronchial arteries are embolized, causing cessation of bleeding. Although rare, bleeding arising directly from the pulmonary circulation can be seen with Rasmussen aneurysms or pulmonary arteriovenous malformations (AVMs).17
If embolotherapy of the bronchial artery is being considered, caution should be exercised because of the potential origin of anterior spinal artery branches from the bronchial circulation. Contributions to the anterior spinal artery from the right bronchial artery are seen in approximately 5% of patients, whereas contributions from the left bronchial artery are decidedly rare.14 Branches supplying the anterior spinal artery are angiographically visualized by a characteristic “hairpin” course that is anterior to the vertebral column; the artery then descends or ascends in the midline. Although embolization procedures can be performed with coaxial catheterization of the bronchial artery beyond the origin of the spinal branches, the possibility of severe cord compromise must be considered before such a procedure is undertaken. Experimental evidence suggests that particles larger than 250 μm are too large to enter small spinal feeders18 and therefore may be used safely in bronchial artery embolization. Some authors advocate that spinal cord infarction is a potential rather than real possibility, with reported cases of transverse myelitis occurring before the advent of nonionic contrast media.19
Benign disease processes that cause bronchial arterial bleeding include infectious causes such as tuberculosis and aspergillosis and noninfectious inflammatory causes such as bronchiectasis, pulmonary sarcoid, and cystic fibrosis.14,17 Chronic inflammatory processes can cause significant recruitment of collateral vessels from the subclavian, axillary, internal mammary, intercostal, and phrenic arteries.20 In the setting of persistent or recurrent hemoptysis following successful bronchial artery embolization, repeat diagnostic angiography as well as embolotherapy of other potential collateral vessels should be undertaken. Some authors advocate bronchial artery embolization as a preemptive therapy in patients with cystic fibrosis before they develop potentially massive hemoptysis.21 The embolic material of choice in patients with enlarged bronchial vessels and chronic inflammatory processes is PVA particles larger than 250 μm but not large enough to occlude the vasculature at the proximal artery level. These agents embolize distal enough to preclude significant collateral vessel recruitment while allowing repeat embolotherapy to be performed if needed. Larger permanent agents such as coils are contraindicated because repeat embolotherapy will be difficult, if not impossible, because of the proximal vessel occlusion. Success rates for hemostasis following bronchial artery embolization range from 77% to 91%.13–15,17 The success rates do not appear to differ significantly among the various inflammatory causes.
Trauma
In addition to inflammatory causes of bleeding, embolization may be used in patients presenting with hemorrhage following trauma; However, in the setting of trauma the underlying nature of the vessels as well as the desired endpoint differ greatly from those seen in the setting of chronic inflammatory disease. In most instances, posttraumatic bleeding involves previously healthy vessels that undergo acute changes such as transection or pseudoaneurysm formation; temporizing measures are frequently all that are required to allow the vessel to undergo the healing process or to stabilize the patient preoperatively. Because of these differences, the embolic agents differ from those seen with hemorrhage from chronic inflammatory diseases.
Hemorrhage associated with pelvic fractures is one of the most frequent traumatic settings in which embolotherapy is used. Fractures in which the constraining ligaments of the pelvis are damaged are most likely to cause severe hemorrhage; placement of an external orthopedic fixation device stabilizes the pelvis in addition to stopping venous, osseous, and minor arterial hemorrhage.22 External fixation devices, therefore, are recommended in patients who are hemodynamically stable and in whom arteriography and embolotherapy are not considered immediately necessary for patient survival. In patients in whom persistent bleeding is suspected following external fixation, diagnostic angiography is necessary to exclude an arterial source of hemorrhage. It is estimated that approximately 7% to 11% of patients with pelvic fractures will require embolization.22
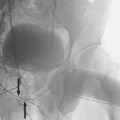
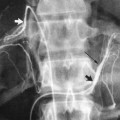
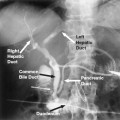
The most common sources of arterial hemorrhage following pelvic trauma are branches of the internal iliac arteries (Fig. 8-1). Cut-film angiography is the preferred method for initial diagnostic angiography done because of the presence of bowel motion artifact in the pelvis during digital subtraction angiography (DSA), although DSA may be sufficient in patients who are hemodynamically unstable and in whom time is crucial. It is recommended that distal aortogram be done before selective catheterization of the internal iliac arterie is performed because of the possibility of concomitant arterial damage to other vessels,22,23 followed by selective angiography of both internal iliac arteries. In most cases, temporary occlusion to stop active extravasation is sufficient, because arterial spasm, thrombosis, and possibly arterial repair will occur once the patient is hemodynamically stable. Because of the rich collateral network within the pelvis, tissue infarction is an uncommon complication of embolization with large agents. Although coils have been used with success, permanent occlusion usually is not considered necessary,23,24 and Gelfoam pledgets are the embolic material of choice.22 Preemptive embolization of arterial branches that are transected but not actively bleeding, as evidenced angiographically by complete occlusion secondary to spasm, is advocated by some authors because the likelihood of delayed hemorrhage is significant in this setting.22 Finally, many trauma patients are hypothermic and therefore coagulopathic, delaying clot formation and possibly allowing retrograde collaterals to open and bleed.25
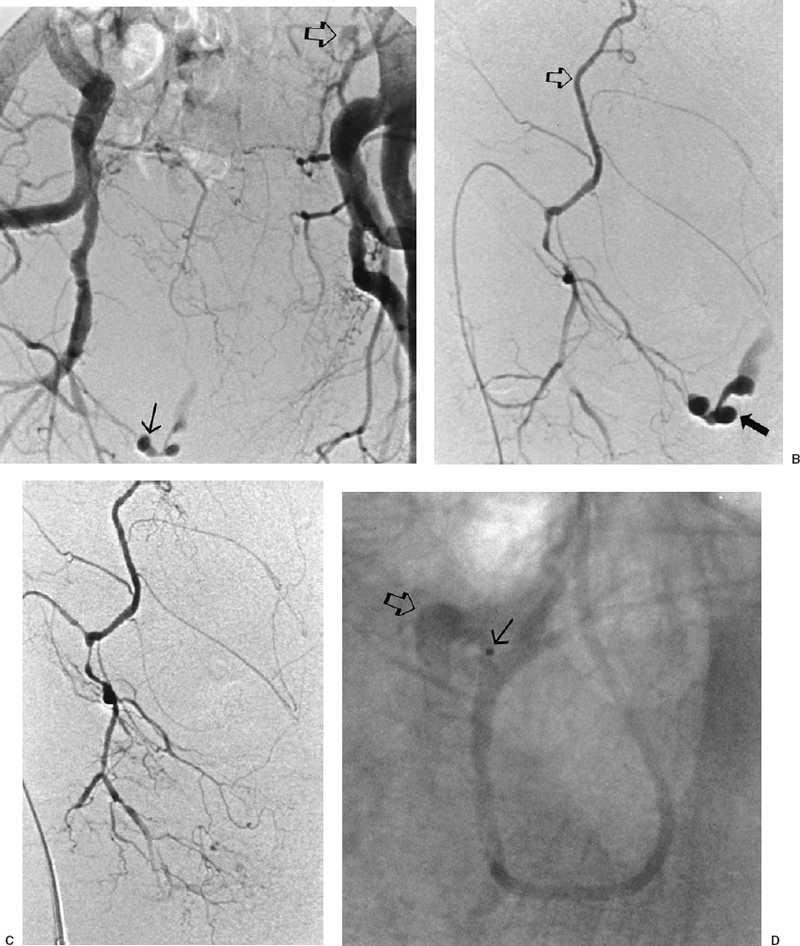
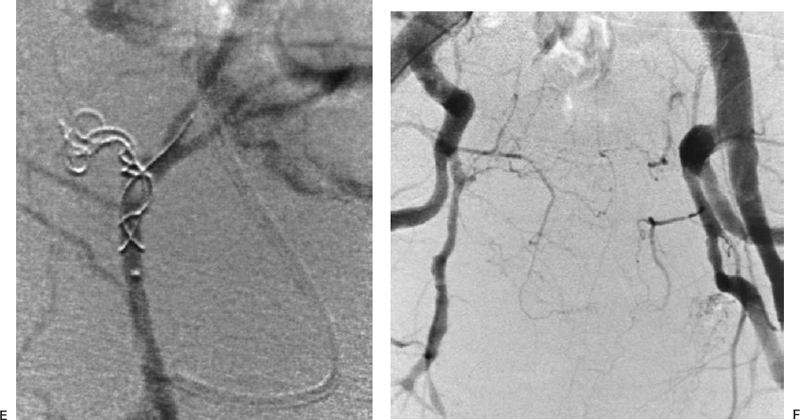
FIGURE 8-1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree