Gestational trophoblastic disease (GTD) is a term for an interrelated group of uncommon conditions derived from gestational placental trophoblasts. GTD affects women during their reproductive years.
Gestational trophoblastic neoplasia (GTN) is a term reserved for cases of persistent human chorionic gonadotropin (hCG) after evacuation of or treatment for GTD.
GTD is an abnormal proliferation of pregnancy-associated placental trophoblastic tissue with malignant potential and abnormal fetal placental development. It arises primarily from abnormal placental tissue, mainly as a result of abnormal fertilization. Although confusing, GTD and GTN are sometimes used interchangeably as a term applied to a broad range of premalignant and malignant conditions, including hydatidiform mole (partial or complete), invasive mole, choriocarcinoma, and placental site trophoblastic tumor (PSTT).
Treated and curable GTD (primarily hydatidiform molar pregnancy) can be separated from more serious and life-threatening persistent trophoblastic neoplasia (PTN). The latter includes invasive mole, choriocarcinoma, and the rare PSTT (PTN has also been referred to by some authors as gestational trophoblastic tumor).
Disease
Molar pregnancy can be divided into complete and partial mole. It can be further characterized as noninvasive versus invasive mole.
Definition
Hydatidiform mole is more commonly known as molar pregnancy and can be complete and partial. It can also be divided into simple (confined to endometrium) versus invasive (invading myometrium or further).
Hydatidiform mole is the result of abnormal proliferative hyperdevelopment of the trophoblast tissue, forming swollen and vesicular chorionic villi. These secrete an excess of hCG, generally after 13 weeks of gestational age. The embryo fails to develop normally, which usually results in spontaneous abortion. Hypertrophied villi may remain in the decidua and continue to secrete hCG and may invade, forming invasive mole. The abnormal tissue may also undergo malignant transformation to form choriocarcinoma.
In the classic presentation of molar pregnancy, quantitative analysis of hCG shows hormone levels greater than expected (blood and urine) in a normal pregnancy at the same stage (especially after 13 weeks of gestation). Serial determination of hCG will indicate rapidly mounting levels that increase greater than during normal single or multiple pregnancy.
Of hydatidiform molar pregnancy, complete hydatidiform mole (CHM) is by far most common form of GTD, followed by partial hydatidiform mole (PHM). CHM and PHM together account for 80% of all GTD. These are generally considered the benign form of GTD, although either can become malignant.
CHM is an abnormal placental cystic mass within the endometrium. If less than 13 weeks of gestation, imaging findings often appear as an anembryonic pregnancy.
Complete molar pregnancies are associated with absence of a fetus or fetal tissue. Rarely, however, complete moles can coexist with a normal pregnancy (i.e., dizygotic twin pregnancy with one normal fetus and one CHM). In molar pregnancies with a coexistent twin, the normal fetus has a normal placenta (i.e., when present, a normal fetus and placenta are seen separate from the molar gestation).
PHM is much less frequent than CHM. PHM has fetal tissue, as an abnormal fetus and/or associated with fetal demise. If a live fetus is present, there is marked associated intrauterine growth restriction. It is rare, but possible, for a partial mole to coexist with a normal pregnancy as well.
Invasive hydatidiform mole (IHM) is histologically identical to the molar pregnancy it was derived from, but invades the myometrium.
Prevalence and Epidemiology
CHM pregnancy is not an uncommon complication of gestation, occurring in one of every 1000 to 2000 pregnancies. CHM is the most common form of GTD. CHM can occur at any maternal age but is most common in younger women (late teens) and those of advanced maternal age (ages 40 to 50). Hydatidiform molar pregnancy is more common in Asian countries than in the United States.
PHM pregnancy is much less frequent than CHM and constitutes 15% to 30% of molar pregnancy.
IHM results from CHM in approximately 15% of cases, whereas only 2% to 3% of partial moles become invasive. Of the molar pregnancies that become invasive, as many as 5% become choriocarcinoma.
Etiology and Pathophysiology
CHM results from paternal disomy (two sets of paternally derived chromosomes). This occurs when both sets of homologous chromosomes are derived from the father, either from dispermy or double fertilization of an empty oocyte by sperm. Therefore complete moles contain 100% paternal genetic makeup. Cytogenetically, 46,XX is the most common karyotype (90%). There is a 1% to 2% CHM recurrence risk in subsequent pregnancy.
CHM is characterized by proliferation and edema within the villous stroma within the trophoblastic tissue. Cell-free fluid-filled cisterns develop. This extends to all villous surfaces (as opposed to normal placental tissue, where just the tips of the anchoring villi within are proliferative).
In a PHM, fetal tissue cytogenetically is most commonly the triploid karyotype. Triploidy results from three complete sets of chromosomes (this can be diandy with two paternal and one maternal set or digyny with two maternal and one paternal set). Rarely, tetraploid or higher karyotypes with multiple sets of paternal chromosomes can combine with one set of maternal chromosomes. There are less hypertrophied trophoblastic villi associated with partial mole as opposed to CHM. Because partial moles are associated with fetal parts, the villi will also commonly contain capillaries and blood cells.
Invasive moles were previously referred to as chorioadenoma destruens and malignant mole. Of molar pregnancies that go on to invasive moles, 50% arise from previous CHM pregnancy, 25% of invasive moles arise from abortion, and 25% arise from normal pregnancy or ectopic pregnancy.
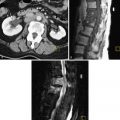
Invasive moles are histopathologically identical to hydatidiform mole, but invasion of the myometrium with necrosis or hemorrhage is present. Invasive moles show excessive trophoblastic overgrowth and deep myometrial penetration by trophoblastic elements (whole villi). They can extend beyond the myometrium to penetrate the peritoneum or extend adjacent to the parametrial region or vaginal vault.
Invasive mole can metastasize (primarily to the lungs).
Manifestations of Disease
Clinical Presentation
The clinical course for GTN and GTD is determined by the patient’s serum hCG levels (curve) after evacuation of molar pregnancy. In 80% of patients, the levels will steadily drop back to normal range within 8 to 12 weeks. In the 20% that go on to malignant hydatidiform mole (invasive mole or choriocarcinoma), the serum hCG levels plateau or increase (an intervening pregnancy must be excluded).
The clinical presentation for complete molar pregnancy includes markedly elevated hCG, irregular vaginal bleeding (most commonly fourth or fifth month of gestation), vaginal passage of vesicles (clusters of cystlike material), spontaneous abortion of the mole, nausea and vomiting or hyperemesis, hypertension before 24 weeks, preeclampsia, painful ovarian cysts (theca lutein cysts), rapidly enlarging pelvic mass, uterine fullness, and/or pelvic swelling. Lung metastasis is present in 4% to 5% of complete moles at the time of diagnosis (rarely with partial moles).
The clinical presentation of partial molar pregnancy is usually much less dramatic than that of a complete mole and includes clinical symptoms mimicking a missed abortion, normal or slightly elevated hCG levels, and spontaneous abortion of malformed fetus. The placenta has a variable appearance but can be cystic. Partial moles are most likely to be confused with complete mole with dizygotic twin by sonography. Partial moles have a possible but decreased propensity to become malignant (2% to 3% as opposed to 15% to 20% with complete moles).
Invasive moles commonly locally invade but are less likely to metastasize (unlike choriocarcinoma). Chorionic villi are found within the myometrium or vascular spaces. The hCG titers are high. When the hCG titers are less than 700 mIU/mL, imaging is often negative (occult). The villi may embolize to the lung, vagina, or brain. If embolization occurs, deposition is prone to hemorrhage from the vascular tissue and may cause hemoptysis, abdominal pain, hematuria, and neurologic symptoms.
Laboratory Studies
Quantitative serum hCG is used to assess for molar pregnancy after 13 weeks and also is used as surveillance to assess response to therapy and disease status.
Liver and renal function tests may also be indicated to assess for metastatic invasive mole or for choriocarcinoma, which can metastasize to these locations.
For patients with a molar pregnancy and a gravid uterine size of more than 16 weeks, obtaining an arterial blood gas value may also be advised because of an increased risk of trophoblastic embolization with acute pulmonary insufficiency.
Imaging
Transvaginal ultrasonography is the best imaging tool for characterizing molar pregnancy and invasive molar disease. Careful endometrial assessment is necessary in threatened abortion cases as to not overlook molar tissue.
Pelvic sonography or magnetic resonance imaging (MRI) follow-up evaluation is important if hCG levels increase after treatment for GTD (to assess for residual and invasive disease progression).
Imaging Algorithm and Findings
Radiography
Plain chest films are warranted with tissue diagnosis of invasion or choriocarcinoma to assess for pulmonary metastases. Standard two-view chest radiography (posteroanterior [PA] and lateral) is suggested.
Ultrasound
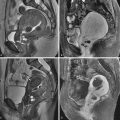
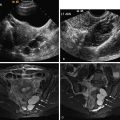
Transvaginal ultrasound (US) is the initial imaging examination of choice. After 13 weeks of gestation, US generally shows complete hydatidiform molar gestations as regions of thickened and hyperechoic tissue with anechoic cystic change. This is the so-called “Swiss cheese” endometrium. The abnormal tissue fills the endometrium and distends the cavity. The anechoic cystic spaces are of variable size between 1 and 30 mm. No embryonic tissue is seen with complete moles (unless coexistent twin fetus is present, as noted previously) ( Figures 24-1 and 24-2 ). There is often concomitant hemorrhage (adjacent sonolucent hematoma or hemorrhagic foci within the larger mass) (see Figure 24-1 ).


Early in pregnancy, CHM can have variable sonographic appearance. Only 50% of patients have endometrial cysts. As stated, CHM can appear identical to an anembryonic pregnancy up to 13 weeks of gestation (i.e., 10-mm gestational sac by transvaginal imaging without yolk sac or 18-mm gestational sac without living embryo). Additionally, CHM can have β-hCG levels in a normal range up to 13 weeks. After 13 weeks the CHM will occupy the central cavity by sonography and appear as a mass with anechoic cystic vesicles developing and resembling a cluster of grapes. See Figure 24-3 .
Partial moles can have fetal tissue or parts on sonography. When present, the fetal tissue frequently has multiple anomalies. When there is live fetal tissue, severe intrauterine growth restriction is common. There is a variable sonographic appearance to the placenta depending on the triploidy (generally cystic placenta if extra paternal chromosomes or small placenta if extra maternal chromosomes).
With US the ovaries can show theca lutein cysts in 50% of molar pregnancy cases secondary to hyperstimulation of ovarian lutein elements by hCG. These are enlarged bilateral multiseptated ovarian cysts ( Figures 24-4 and 24-5 ). They rarely occur before 13 weeks of gestation. Theca lutein cysts enlarge the ovaries and can hemorrhage (see Figure 24-4 ). There is a higher associated risk ovarian torsion.


In CHM, color Doppler imaging will show a cavitary vascular mass with high velocity, low impedance flow on pulsed Doppler (mean resistive index [RI], <0.55) (see Figures 24-2 and 24-7 ).




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree