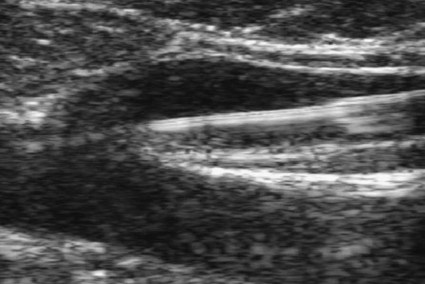
Chapter 106 Robert J. Min and Neil M. Khilnani Chronic venous insufficiency (CVI) is extraordinarily common, with estimates of up to 25% of women and 10% of men suffering from some form of CVI.1 Most patients with CVI have symptoms that interfere with daily living (e.g., leg aches, fatigue, throbbing, heaviness, night cramps). Severe cases can lead to skin damage resulting from chronic venous hypertension (e.g., eczema, edema, hyperpigmentation, lipodermatosclerosis). The majority of patients with leg ulceration have superficial venous insufficiency (SVI) as the primary underlying cause, with SVI being the sole factor in 20%.2 Patients with symptoms typical of CVI and clinical signs of CVI require further evaluation with duplex ultrasound (DUS).3,4 The goal of DUS evaluation is to map out all the incompetent venous pathways responsible for the patient’s condition, including the primary or highest points of reflux and the presence of obstruction.3 Such a map is necessary to determine the best treatment plan. Dr. Boné first reported on delivery of endoluminal laser energy in 1999.5 Since then, a method for treating the entire incompetent vein segment has been described by Min and Navarro.6–8 Endovenous laser treatment, which received approval by the U.S. Food and Drug Administration (FDA) in January 2002, achieves nonthrombotic vein occlusion by delivery of laser energy directly into vein walls. Lasers with wavelengths of 810, 940, 980, 1064, and 1320 nm have all been used with success. Contact between the laser fiber and vein wall is necessary to cause sufficient damage to the vein to result in acute wall thickening with eventual vein contraction and fibrosis. The equipment necessary to perform endovenous laser ablation includes but is not limited to: A 5F vascular introducer sheath with markings is inserted over a guidewire into the vein and passed through the entire abnormal segment into a more central vein. A bare-tipped laser fiber is inserted into the sheath. The sheath is then pulled back to expose the tip of the fiber, and the fiber is locked in place. Under ultrasound guidance, the introducer sheath and fiber are withdrawn out of the deep veins and positioned within the superficial venous system at the junction, as seen in Figure 106-1. The fiber is left in this position during tumescent anesthetic administration and will be repositioned just before delivery of laser energy. Confirmation of position can be made by direct visualization of the red aiming beam through the skin. After administration of tumescent anesthesia, ultrasound is used to check for adequacy. A centimeter halo of fluid surrounding the target vein or separating the vein from the overlying skin has been found to be sufficient, as demonstrated in Figure 106-2. Proper delivery of tumescent fluid may be particularly useful for maximizing procedural safety when performing endovenous laser therapy in certain locations such as tributaries close to the skin, the SSV near the saphenopopliteal junction, or the GSV below the knee, owing to the close proximity of nerves or arterial branches. Adequacy of separation of arterial branches from the target vein can be checked with color Doppler ultrasound.
Great Saphenous Vein Ablation
Equipment
Technique
Technical Aspects
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Great Saphenous Vein Ablation

 -inch needle
-inch needle