, Joungho Han2, Man Pyo Chung3 and Yeon Joo Jeong4
(1)
Department of Radiology Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South Korea)
(2)
Department of Pathology Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South Korea)
(3)
Department of Medicine Division of Pulmonary and Critical Care Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South Korea)
(4)
Department of Radiology, Pusan National University Hospital, Busan, Korea, Republic of (South Korea)
Abstract
Ground-glass opacity (GGO) appears at thin-section CT (TSCT) as hazy increased opacity of the lung, with the preservation of bronchial and vascular margins. It is caused by partial filling of airspaces; interstitial thickening due to fluid, cells, or fibrosis; partial collapse of alveoli; increased capillary blood volume; or combination of these, the common factor being the partial displacement of air [1]. The GGO is less opaque than consolidation, in which bronchovascular margins are obscured (Fig. 20.1).
Ground-Glass Opacity with Reticulation and Fibrosis
Definition
Ground-glass opacity (GGO) appears at thin-section CT (TSCT) as hazy increased opacity of the lung, with the preservation of bronchial and vascular margins. It is caused by partial filling of airspaces; interstitial thickening due to fluid, cells, or fibrosis; partial collapse of alveoli; increased capillary blood volume; or combination of these, the common factor being the partial displacement of air [1]. The GGO is less opaque than consolidation, in which bronchovascular margins are obscured (Fig. 20.1).
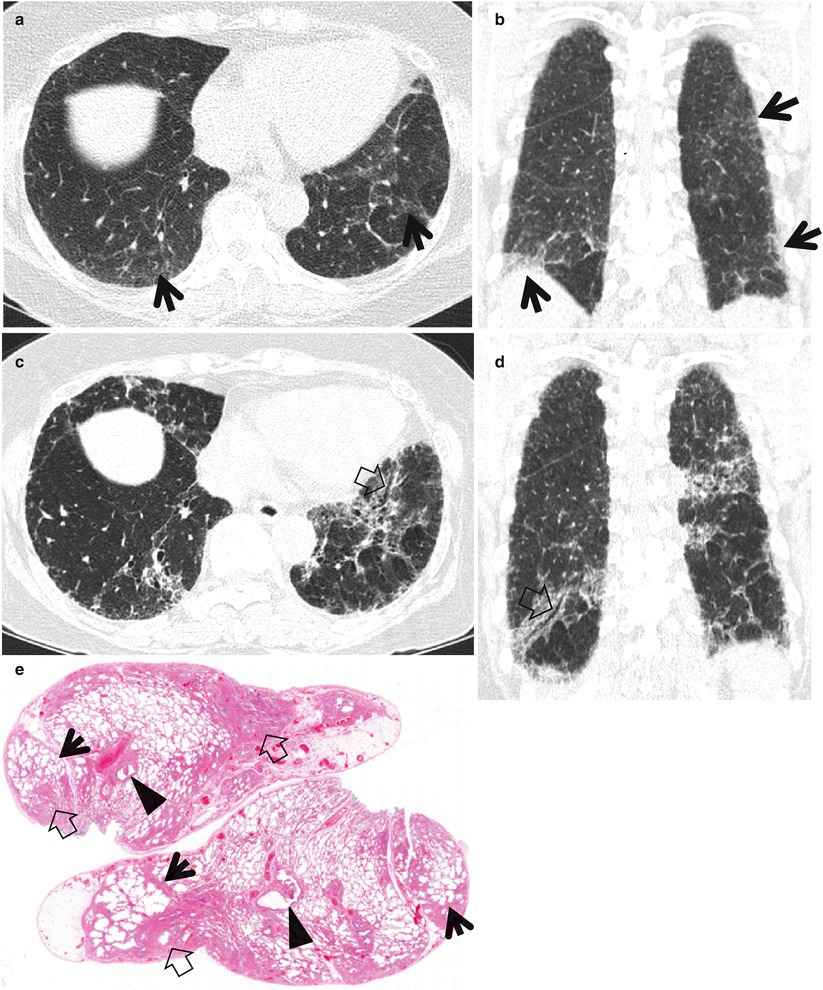

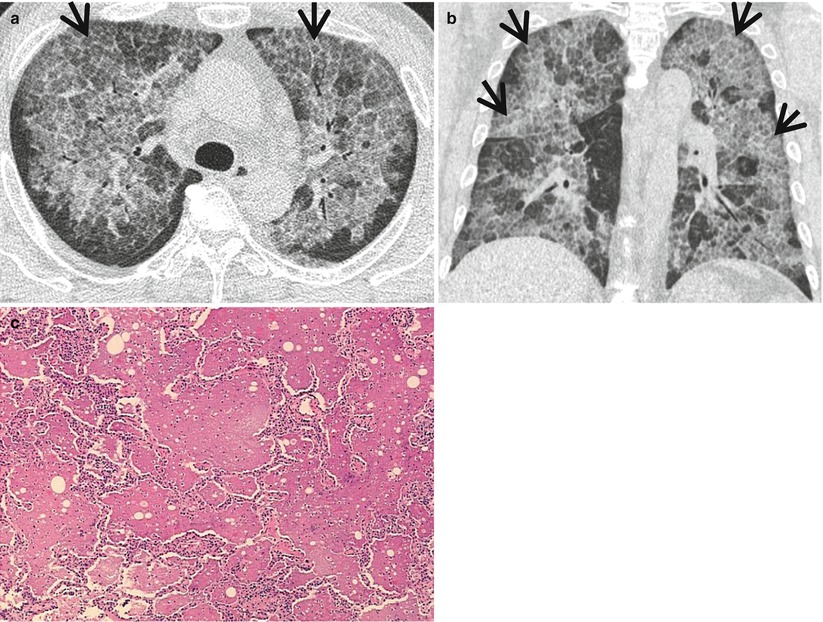
Fig. 20.1
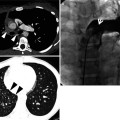
Usual interstitial pneumonia manifesting as subpleural patchy areas of reticulation and ground-glass opacity without honeycombing cyst in a 73-year-old man. (a) Lung window image of thin-section (1.0-mm section thickness) CT scan obtained at level of liver dome shows patchy areas of reticulation and ground-glass opacity (arrows) in bilateral lower lung zones. (b) Coronal reformatted image (2.0-mm section thickness) also shows patchy areas of reticulation and ground-glass opacity (arrows) in lower lung zones. (c) Three-year follow-up CT demonstrates increased extent of reticulation and ground-glass opacity in both lungs. Also note traction bronchiectasis (open arrow). (d) Coronal reformatted image (2.0-mm section thickness) also shows increased extent of reticulation and ground-glass opacity in both lungs. Also note traction bronchiectasis (open arrow). (e) Low-magnification (×10) photomicrograph of surgical biopsy specimen obtained from right lower lobe and at follow-up CT acquisition time discloses heterogeneous areas of inflammation (arrows) and fibrosis (open arrows) in alveolar walls in terms of time and region. Also note airway wall thickening and dilatation (arrowheads)
Reticulation at TSCT includes interlobular septal thickening, intralobular lines, or the cyst walls of honeycombing (HC) [2]. When GGO lesions were admixed with reticulation, traction bronchiectasis, and architectural distortion, the lesions represent histologically the area of pulmonary fibrosis.
Diseases Causing the Pattern
Usual interstitial pneumonia (UIP) (Fig. 20.2) and fibrotic or mixed fibrotic and cellular nonspecific interstitial pneumonia (NSIP) (Figs. 20.3 and 20.4) are the two most common diseases that show GGO mixed with reticulation with or without honeycombing.
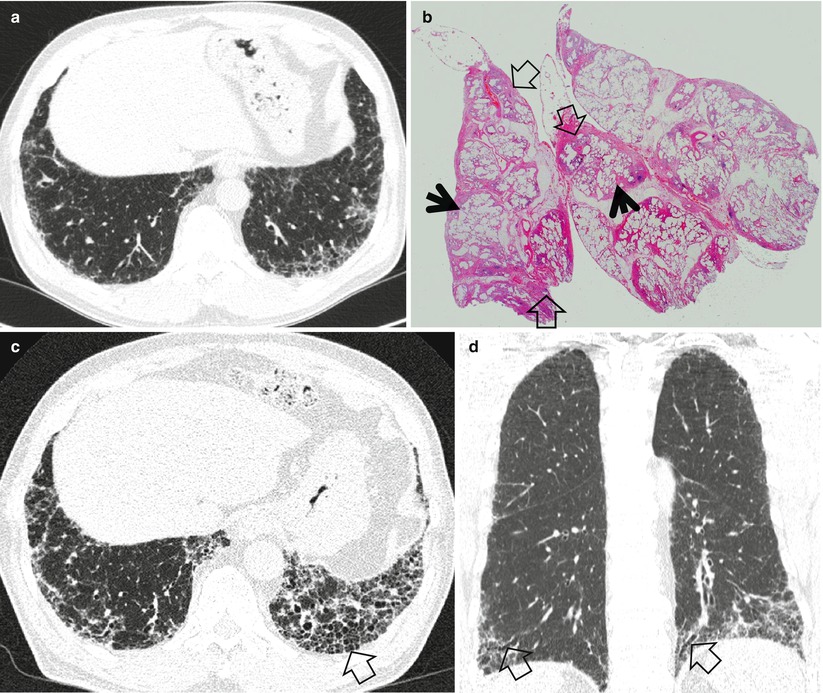
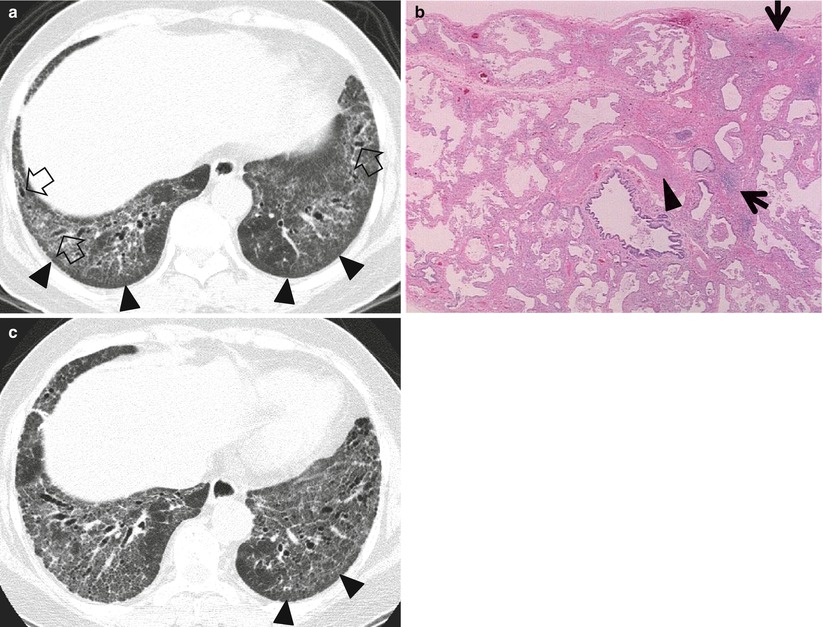
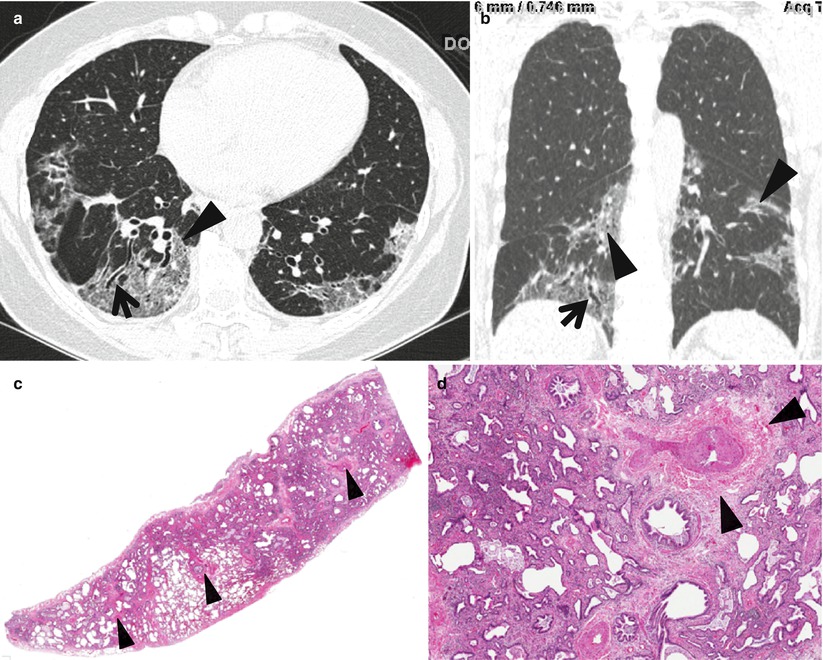

Fig. 20.2
Usual interstitial pneumonia manifesting as subpleural patchy areas of reticulation and ground-glass opacity without honeycombing cyst in a 62-year-old man. (a) Lung window image of thin-section (1.5-mm section thickness) CT scan obtained at level of liver dome shows patchy areas of reticulation and ground-glass opacity in bilateral lower lung zones. (b) Low-magnification (×4) photomicrograph of surgical biopsy specimen obtained from left lower lobe discloses heterogeneous areas of inflammation (arrows) and fibrosis (open arrows) in alveolar walls in terms of time and region. (c) Seven-year follow-up CT demonstrates increased extent of reticulation in both lungs. Also note new area of honeycombing (open arrow). (d) Follow-up CT in coronal reformatted view depicts reticulation predominant abnormalities with lower lung zone predominance. Also note traction bronchiectasis (open arrows)

Fig. 20.3
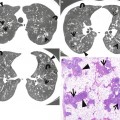
Fibrotic nonspecific interstitial pneumonia in a 50-year-old woman. (a) Lung window image of thin-section (1.0-mm section thickness) CT scan obtained at level of liver dome shows patchy areas of reticulation and ground-glass opacity in bilateral lower lung zones. Subpleural sparing (arrowheads), one of important signs that help differentiate usual interstitial pneumonia from this disease on CT, is clearly seen. Also note traction bronchiectasis (open arrows). (b) Low-magnification (×40) photomicrograph of surgical biopsy specimen obtained from right lower lobe discloses clearly temporal and regional homogeneity; namely, whole alveolar wall shows fibrotic changes with little lymphocytic infiltration. Also note few areas (arrows) of lymphocytic infiltration and thickened vessel walls (arrowhead). (c) Four-year follow-up CT demonstrates slightly increased extent of reticulation in both lungs. Still subpleural sparing (arrowheads) is overt particularly in left lung

Fig. 20.4
Fibrotic nonspecific interstitial pneumonia in a 60-year-old woman. (a) Lung window image of thin-section (1.0-mm section thickness) CT scan obtained at level of cardiac ventricle shows patchy areas of ground-glass opacity and reticulation. Please note involvement of central portion (arrowhead) of lung as well as subpleural lungs, which is somewhat more peculiar to nonspecific interstitial pneumonia than usual interstitial pneumonia. Also note traction bronchiectasis (arrow). (b) Coronal reformatted imaged demonstrates central involvement (arrowheads) involvement of lung lesions along bronchovascular bundles, not to mention of subpleural lung involvement. Also note traction bronchiectasis (arrow). (c) Low-magnification (×4) photomicrograph of surgical biopsy specimen obtained from right lower lobe exhibits uniform interstitial expansion with fibrosis and little lymphocytic infiltration. Also note thickened vessel walls (arrowheads). (d) Somewhat closer look (×40) of surgical biopsy specimen discloses clearly temporal and regional homogeneity, namely, uniform interstitial expansion due to fibrosis. Also thickened vessel wall, which is postinflammatory changes (arrowheads)
Distribution
Subpleural and basal predominance is one of the four major prerequisites (subpleural and basal predominance, reticular abnormality, HC with or without traction bronchiectasis, and absence of features listed as inconsistent with UIP pattern) to be called UIP pattern at TSCT [3]. The TSCT features that help differentiate best NSIP from UIP or chronic hypersensitivity pneumonitis are relative subpleural sparing of GGO and reticulation [4] (Fig. 20.3). NSIP may also demonstrate bronchovascular distribution of lung lesions and subpleural distribution as well (Fig. 20.4).
Clinical Considerations
Patients with UIP or fibrotic NSIP who have a high fibrotic score (the extent of reticulation plus HC) at TSCT and a low DLco level appear to have a high death risk [5]. Even in cases of UIP and fibrotic NSIP with little HC, serial CT discloses an increase in the extent of HC and reticulation and a decrease in the extent of GGO. Overall extent of lung fibrosis (reticulation and HC) at the baseline CT examination appears predictive of survival in UIP and fibrotic NSIP with little HC [6].
Key Points for Differential Diagnosis
Diseases | Distribution | Clinical presentations | Others | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Zones | ||||||||||||
U | M | L | SP | C | R | BV | R | Acute | Subacute | Chronic | ||
IPF/UIP | + | + | + | + | + | |||||||
NSIP | + | + | + | + | + | + | + | + | Female predominance; subpleural sparing and along BV bundles | |||
Honeycombing with Subpleural or Basal Predominance Chap. 17.
Ground-Glass Opacity with Reticulation, but without Fibrosis (Crazy-Paving Appearance)
Definition
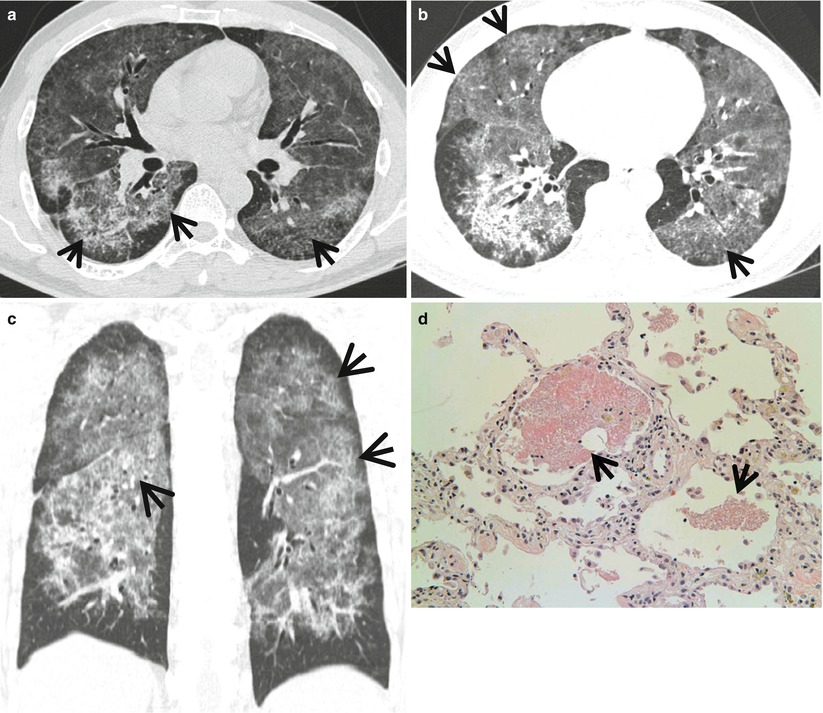
The crazy-paving appearance consists of a network of a smooth linear pattern superimposed on the area of ground-glass opacity (GGO) on high-resolution CT scans (Fig. 20.5). Once thought as specific finding for pulmonary alveolar proteinosis (PAP), currently the crazy-paving appearance is a nonspecific finding seen in a variety of interstitial and airspace lung diseases [7].
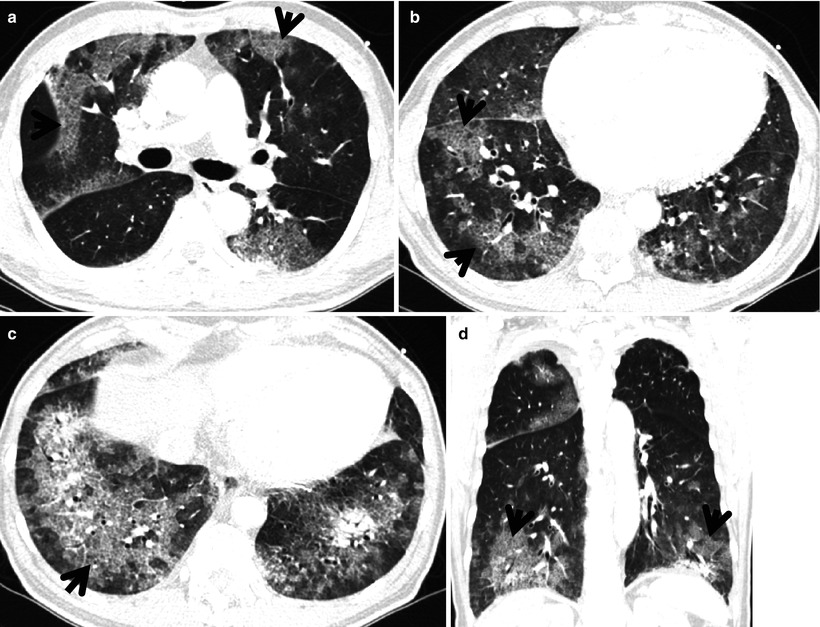

Fig. 20.5
Diffuse alveolar hemorrhage manifesting as crazy-paving appearance at thin-section CT in a 60-year-old man who had ischemic cardiomyopathy and received heparin and warfarin therapy. (a–c) Lung window images of CT scans (2.5-mm section thickness) obtained at levels of main bronchi (a), cardiac ventricle (b), and liver dome (c), respectively, show patchy and extensive areas of ground-glass opacity containing internal reticulation (crazy-paving appearance, arrows) in both lungs. Subpleural sparing is overt in all lung zones. (d) Coronal reformatted image (2.0-mm section thickness) also demonstrates crazy-paving appearance (arrows) in both lungs with subpleural sparing
Diseases Causing the Pattern
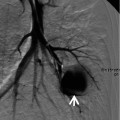
The diseases causing crazy-paving pattern include acute bacterial, viral, or Pneumocystis pneumonia (Fig. 20.6), acute interstitial pneumonia (AIP) or adult respiratory distress syndrome (ARDS), diffuse alveolar damage superimposed on usual interstitial pneumonia (UIP), diffuse alveolar hemorrhage (DAH) (Fig. 20.5), pulmonary interstitial edema, pulmonary alveolar proteinosis (PAP) (Fig. 20.7), nonspecific interstitial pneumonia (NSIP), subacute hypersensitivity pneumonitis (HP), organizing pneumonia, sarcoidosis, lipoid pneumonia (Fig. 20.8), and diffuse form of mucinous or nonmucinous adenocarcinoma (AIS, former bronchioloalveolar carcinoma [BAC]) or lung adenocarcinoma [7, 8] (Fig. 20.9).
Get Clinical Tree app for offline access

Fig. 20.6
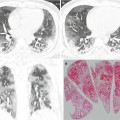
Pneumocystis jirovecii pneumonia in a 48-year-old man with asthma who received corticosteroid therapy. (a, b) Lung window images of CT scans (2.5-mm section thickness) obtained at levels of right middle lobar bronchus (a) and right inferior pulmonary vein (b), respectively, show diffuse ground-glass opacity harboring internal reticulation (crazy-paving appearance, arrows) in both lungs. Subpleural sparing is seen in all lung zones. (c) Coronal reformatted image (2.0-mm section thickness) also demonstrates crazy-paving appearance (arrows) in both lungs with subpleural sparing. (d) High-magnification (×200) photomicrograph of transbronchial lung biopsy specimen obtained from right lower lobe discloses intra-alveolar foamy exudate (arrows) and mild interstitial inflammation and fibrosis suggesting Pneumocystis pneumonia and acute lung injury

Fig. 20.7
Pulmonary alveolar proteinosis in a 52-year-old man. (a




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree