Evis Sala, Susan Freeman, Susan M. Ascher, Hedvig Hricak
Gynaecological Cancer
The results of diagnostic imaging tests frequently change treatment strategies and affect our understanding of disease processes. This chapter gives a brief review of common gynaecological malignancies and presents indications for each imaging technique used (Table 41-1) while focusing on advances in ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT). As with any changing technological arena, imaging strategies are not static and require ongoing updates and re-evaluation.
TABLE 41-1
The Choice of Imaging Investigation in Endometrial, Cervical and Ovarian Cancer
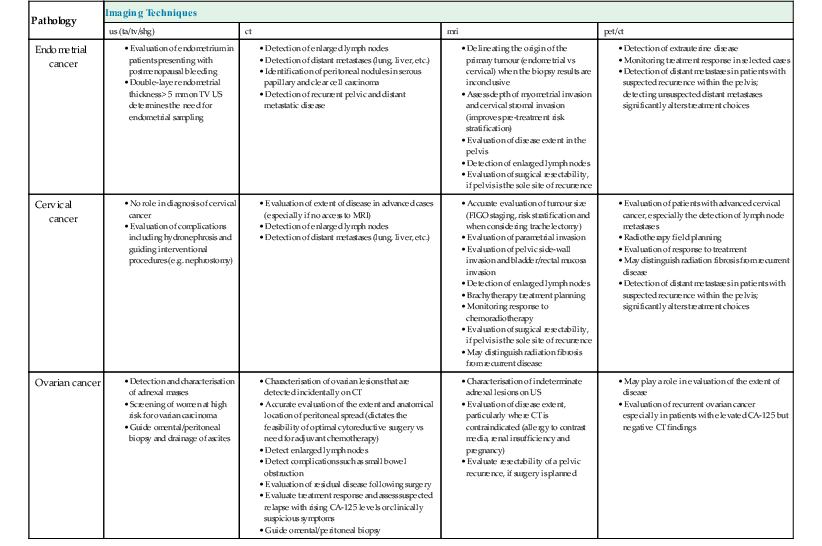
| Pathology | Imaging Techniques | |||
| us (ta/tv/shg) | ct | mri | pet/ct | |
| Endometrial cancer | ||||
| Cervical cancer | • Evaluation of parametrial invasion • Evaluation of pelvic side-wall invasion and bladder/rectal mucosa invasion • Detection of enlarged lymph nodes • Brachytherapy treatment planning • Monitoring response to chemoradiotherapy • Evaluation of surgical resectability, if pelvis is the sole site of recurrence | |||
| Ovarian cancer | ||||
TA, transabdominal; TV, transvaginal; SHG, sonohysterography.
Imaging Techniques
Ultrasound
Ultrasound (transabdominal or transvaginal) is accepted as the primary imaging technique for examining the female pelvis. Currently, the main role of US in gynaecological oncology includes evaluation of a suspected pelvic mass, evaluation of causes of uterine enlargement, identification of endometrial abnormalities in a patient with postmenopausal bleeding and characterisation of ovarian masses. In addition, US has become invaluable in guiding a wide selection of invasive procedures such as transabdominal and transvaginal guidance of fluid or tissue sampling,1 transvaginal-guided drain placement and guidance for placement of brachytherapy devices for cervical and endometrial malignancies.
A full bladder is mandatory for transabdominal US, as it provides a sonic window to image the pelvic organs. It is usual to employ 3.5–5.0 MHz transducers. Transvaginal US, which is optimally performed with an empty bladder, provides greater detail of the anatomy and pathology due to the closer apposition to the pelvic organs, as well as the higher frequencies of insonation (5.0–7.5 MHz). Colour, power and spectral Doppler provide additional information regarding associated vascularity.
Ultrasound has many advantages: it is relatively inexpensive, provides multiplanar views, is widely available and lacks ionising radiation. Its portability allows use in virtually any setting, including the ultrasound suite, operating room, patient bedside or radiotherapy suite. However, US also has a number of limitations: it is operator dependent and image quality varies with patient body habitus. Although transvaginal, sonohysterography and endorectal US provide improved spatial resolution, they are not as useful as either CT or MRI in the staging of pelvic malignancies, including the evaluation of regional extent or metastatic spread.
Computed Tomography
CT is the most commonly used primary imaging study for evaluating the extent of gynaecological malignancies and for detecting persistent and recurrent disease although there is increasing use of MRI. CT-guided biopsy of omental cake or pelvic mass can be used to obtain histological confirmation of ovarian cancer and to confirm pelvic recurrence.1 Advantages of CT include wide availability, fast data acquisition and high spatial resolution. Disadvantages of CT include the use of ionising radiation, degradation of image quality by body habitus or metallic hip prosthesis and the risk of morbidity and mortality associated with iodinated contrast agents. Although CT is useful in the advanced stages of pelvic malignancy, it often has limited utility in characterising early-stage disease.
CT images of the abdomen and pelvis are acquired in the portal venous phase, 70 s following an injection of intravenous low osmolar contrast medium; this enhances blood vessels and viscera, allowing easier identification of enlarged lymph nodes and parenchymal lesions. Oral contrast medium is utilised to opacify the small and large bowel, which allows detection of bowel serosal deposits and the differentiation of bowel loops from pelvic and nodal disease.
Magnetic Resonance Imaging
The role of MRI in the evaluation of gynaecological malignancies has evolved during the past two decades. MRI has been shown to be superior to CT in the work-up of endometrial and cervical cancer and may be a useful problem-solving tool in the evaluation of ovarian cancer. In addition, there is evidence that MRI may aid the differentiation of radiation fibrosis from recurrent tumour. The accuracy of MRI assessment of lymph node invasion is similar to that of CT; both rely primarily on size criteria to detect lymph node metastases.
Although MRI is still relatively expensive, it has been shown to minimise costs in some clinical settings by limiting or eliminating the need for further expensive and/or more invasive diagnostic or surgical procedures. Advantages of MRI include superb spatial and tissue contrast resolution, no use of ionising radiation, multiplanar capability and fast (i.e. breath-hold and breathing-independent) techniques. MRI is the technique of choice for patients with previous reactions to iodinated IV contrast media or impaired renal function. However, MRI is contraindicated in patients with implants such as pacemakers, neural stimulators or cochlear implants, certain vascular clips and metallic objects. Some patients may experience claustrophobia, causing difficulty in completing the examination or requiring sedative premedication in subsequent MR examinations.
The basic imaging protocol for gynaecological MRI includes T1-weighted images (T1WI) of the pelvis in the axial plane and T2-weighted images (T2WI) in the axial and sagittal planes. When high signal intensity is seen within the adnexa on T1WI, which may represent fat or haemorrhage, fat-saturation T1-weighted images (T1W FS) are mandatory to differentiate between the two. Staging of gynaecological malignancies requires large field-of-view axial T1WI and/or T2WI of the pelvis and abdomen to identify enlarged lymph nodes, hydronephrosis and bone marrow changes.2,3 High-resolution, small field-of-view, axial oblique (short-axis) T2W fast spin-echo (T2W FSE) images taken parallel to the short axis of the uterine corpus are essential in evaluating accurate depth of myometrial invasion in endometrial carcinoma. In the assessment of patients with cervical cancer, high-resolution, small field-of-view, axial oblique (short-axis) T2W FSE images parallel to the short axis of the cervix are crucial for identification of parametrial invasion.
Dynamic multiphase contrast-enhanced MRI using a three-dimensional (3D) gradient echo T1WI after intravenous gadolinium is often utilised in staging patients with endometrial cancer; it is also useful in the characterisation of complex adnexal lesions and can aid in the detection of small peritoneal and serosal implants, when ovarian cancer is suspected. Routine use of dynamic multiphase contrast-enhanced 3D T1-weighted sequences is not recommended for staging of patients with cervical carcinoma. However, they may be very useful for accurate delineation of small cervical tumours in patients being considered for fertility sparing surgery (i.e. trachelectomy) and differentiation of tumour recurrence from radiation fibrosis in patients with cervical cancer.
Increasingly, diffusion-weighted MRI (DW-MRI) is routinely incorporated in MRI staging protocols for uterine malignancies. DW-MRI can be useful for accurately determining the depth of myometrial invasion in patients with endometrial cancer, which can be particularly helpful in cases of tumours that are either iso- or hyperintense relative to the myometrium on T2WI or when the use of intravenous contrast medium is contraindicated, and may replace dynamic imaging in the future.4 DW-MRI may also improve detection of drop metastases in the cervix or metastatic foci outside the uterus, such as adnexa or peritoneum.4
Positron Emission Tomography/Computed Tomography
The main applications of 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) PET/CT in gynaecological oncology are the staging of cervical cancer and detection of recurrent ovarian, cervical and endometrial cancer. PET/CT takes advantage of the biochemical changes associated with malignancy that are more specific than, and often precede, the structural changes visualised by conventional means. Specifically, FDG-PET/CT exploits the accelerated rate of glycolysis that is common to neoplastic cells to image tumours. FDG-PET/CT does not have the same spatial resolution or soft-tissue contrast as US or MRI, but image fusion techniques that overlay FDG-PET images onto MR images are available. There are several disadvantages of FDG-PET/CT. False negatives can occur with lesions smaller than 0.5 cm and with certain tumour types that demonstrate low metabolic activity. False-positive results can be seen with inflammatory or infective aetiology, postoperative changes or reactive lymphadenopathy, including chronic inflammatory disorders such as granulomatous disease.
Endometrial Carcinoma
Endometrial cancer is the most commonly diagnosed gynaecological malignancy in affluent societies. Presenting as postmenopausal bleeding, the disease has a peak incidence between the ages of 55 and 65 years. Risk factors include unopposed oestrogen intake, nulliparity, obesity and diabetes. The prognosis of endometrial carcinoma depends on a number of factors, including stage, depth of myometrial invasion, nodal status and tumour grade. Preoperative evaluation of prognostic factors helps in subspecialist treatment planning.
Detection, Diagnosis and Staging
Endometrial carcinoma is typically diagnosed at endometrial biopsy or dilatation and curettage, with imaging being reserved to evaluate the extent of disease.5 Imaging criteria for staging of endometrial cancer are based on the International Federation of Gynecology and Obstetrics (FIGO) surgical-pathological staging system, revised in 2009 (Table 41-2).6 Full FIGO staging comprises a total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), peritoneal washings and retroperitoneal lymph node dissection. Imaging is not part of the FIGO staging system for endometrial cancer; however, both MRI and CT are increasingly playing a crucial role in risk stratification and surgical treatment planning.5
TABLE 41-2
FIGO Staging of Endometrial Carcinoma with Corresponding MR Findings
| FIGO Stage | Description of Stage | MRI Findings |
| Stage I | Tumour confined to the corpus uteri | |
| Ia | Tumour extending to <50% of myometrial depth | Abnormal signal intensity extends into <50% of the myometrium |
| Ib | Tumour extending to ≥50% of myometrial depth | Abnormal signal intensity extends into ≥50% of the myometrium |
| Stage II | Tumour invades cervical stroma, but does not extend beyond the uterus | Disruption of low signal intensity cervical stroma by tumour A widened internal os with tumour protruding into the endocervical canal does not represent stromal invasion |
| Stage III | Local and/or regional spread of the tumour | |
| IIIa* | Tumour invades the serosa of the corpus uteri and/or adnexa | Disruption of continuity of outer myometrium. Irregular uterine configuration |
| IIIb | Vaginal and/or parametrial involvement | Segmental loss of hypointense vaginal wall |
| IIIc | Metastases to pelvic and/or para-aortic lymph nodes | Regional or para-aortic nodes >1 cm in short-axis diameter. Additional suspicious features include multiple small rounded lymph nodes, irregular lymph node contour, abnormal signal intensity similar to that of the primary tumour, presence of necrosis |
| IIIc1 | Positive pelvic nodes | |
| IIIc2 | Positive para-aortic lymph nodes ± positive pelvic lymph nodes | |
| Stage IV | Tumour invades bladder and/or bowel mucosa, and/or distant metastases | |
| IVa | Tumour invades bladder and/or bowel mucosa (biopsy proven) | Abnormal signal intensity disrupts normal low signal intensity bladder/rectal mucosa. Note that bullous oedema does not indicate stage IVa |
| IVb | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes | Tumour in distant sites or organs |
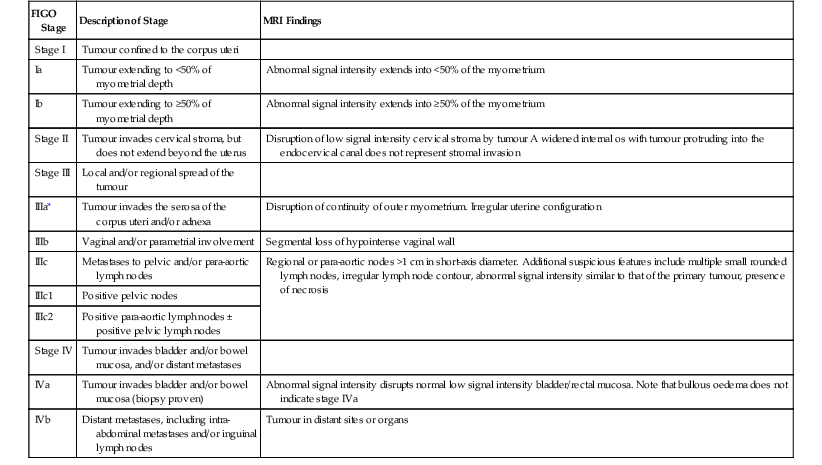
* Positive cytology obtained at peritoneal washings should be recorded but does not alter any stage.
Ultrasound
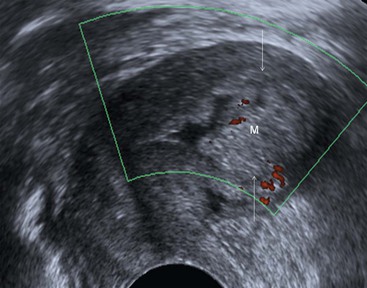
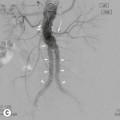
Transvaginal (TVUS) is superior to transabdominal US for imaging endometrial abnormalities.7 The most common appearance of endometrial cancer is non-specific thickening of the endometrium. Endometrial thickening in endometrial carcinoma is indistinguishable from that found with hyperplasia or a polyp. However, the diagnosis of endometrial cancer should be considered when the endometrial/myometrial junction is disrupted or the endometrial surface is irregular (Fig. 41-1). In postmenopausal women, the threshold value for a thickened endometrium is 5 mm.8 In sonohysterography, endometrial cancer may appear as an intracavitary polyp or as asymmetric thickening of the endometrial lining. Doppler, colour and 3D US have been advocated to improve endometrial carcinoma detection.
The use of US is limited to the evaluation of stage I disease with emphasis on the evaluation of the depth of myometrial invasion. For the evaluation of myometrial invasion, the presence and continuity of the hypoechoic halo that surrounds the outer layer of the endometrium are assessed (i.e. intact, focally disrupted or totally disrupted). The extent of myometrial invasion is then estimated by measuring the distance from the central lumen of the uterus to the distal junction between tumour and normal myometrium. Transvaginal ultrasound has been reported to have a sensitivity of 77 to 100%, a specificity of 65 to 93% and an overall accuracy of 60 to 76% in assessing the degree of myometrial invasion.3
Computed Tomography
On contrast-enhanced CT, endometrial cancer is seen as a hypodense mass relative to normal myometrium but delineation of the tumour is difficult as there is relatively little contrast difference between tumour and the myometrium.9
CT is most commonly used in the assessment of advanced disease and performs as well as MRI in identifying extrauterine spread and enlarged lymph nodes.3 Its greatest clinical impact is in confirming parametrial and side-wall extension in stage III tumours and in detecting pelvic lymphadenopathy. Limitations of CT include a tendency to understage endometrial carcinoma because of limited soft-tissue resolution which leads to inaccurate estimation of depth of myometrial invasion. In addition, CT has a limited accuracy in evaluation of cervical stroma invasion and bladder or rectal mucosa invasion.
Magnetic Resonance Imaging
MRI is considered the most accurate imaging technique for preoperative assessment of endometrial carcinoma due to its excellent soft-tissue contrast resolution. The National Cancer Institute in France10 has recently incorporated MRI into the standard preoperative assessment of patients with endometrial carcinoma. The European Society of Urogenital Imaging (ESUR) and the Royal College of Radiologists cancer imaging guidelines also recommend MRI for staging of high-grade endometrioid adenocarcinomas (type I histology) as well as serous papillary and clear cell adenocarcinomas (type II histology).11
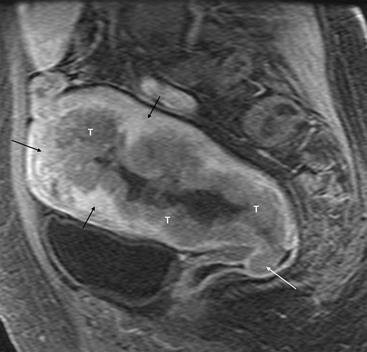
Although endometrial cancer may demonstrate high signal intensity on T2-weighted sequences, it is more typically heterogeneous and may even be of low signal intensity. Following IV contrast medium administration, there is early avid enhancement of normal myometrium. Endometrial cancer enhances more slowly than the adjacent myometrium, allowing identification of small tumours, even those contained by the endometrium.12 In the later phases of enhancement, tumour appears hypointense relative to the myometrium (Fig. 41-2).
MRI is significantly superior to US and CT in the evaluation of both depth of myometrial invasion and tumour invasion into the cervical stroma. The overall staging accuracy of MRI has been reported to be between 85 and 93%.