Introduction
The region of the head and neck involves complex anatomic structures, including the skull base, temporal bone, orbit, oral cavity, paranasal sinuses, and aerodigestive tract. Various kinds of pathology, including trauma, infection, congenital disease, and benign and malignant tumors develop in this area. Among these, evaluation of malignant tumors is one of the most common and most important imaging challenges in head and neck imaging.
Malignancies of the head and neck include cancers of the mucosal surface (epithelium), salivary glands, thyroid, skin, soft tissues, and lymphoid structures. Squamous cell carcinoma is the most common subtype of head and neck cancer and may arise in the sinonasal cavity and aerodigestive tract. Oral cavity and pharyngeal cancer are the eighth most common cancer among men in the United States, and is twice as common in men as in women ( ). Squamous cell carcinoma accounts for more than 90% of oropharyngeal cancers ( ). Consequently, the term head and neck cancer is often used synonymously with head and neck squamous cell carcinoma (HNSCC) arising from the mucosal epithelium of the sinonasal tract, oral cavity, pharynx, and larynx ( ).
Two-thirds of cases of HNSCC present with locally advanced disease and/or cervical lymph node metastases. The five-year survival rate of head and neck cancer patients ranges from 40% to 70%, depending on initial staging. Distant metastases are also frequent, most commonly affecting the lungs and bones ( ).
Risk factors for head and neck cancer include genetic traits, smoking, alcohol, and infectious agents ( ). Of note, the incidence of oropharyngeal cancer associated with human papillomavirus (HPV) has significantly increased throughout the world ( ). Oropharyngeal cancers that contain HPV DNA (p16-positive) have significantly better outcomes than their counterparts without HPV DNA, which is reflected in the eighth edition of the American Joint Committee (AJCC) Cancer Staging Manual ( ; ).
HNSCC is often initially suspected by history and physical examination, including direct visualization of the primary tumor and palpation of metastatic lymph nodes. Definite diagnosis is usually made by biopsy. Given the close proximity of critical structures in the head and neck, pretreatment imaging is crucial for radiation planning ( ). The AJCC eighth edition introduced new imaging parameters for staging, including depth of invasion for T staging of oral cavity cancer, and perinodal extension for nodal staging of oropharyngeal cancer ( ).
Current role of imaging in patients with known or suspected head and neck cancer
Computed tomography
While computed tomography (CT) and magnetic resonance imaging (MRI) are both suitable imaging modalities for staging aerodigestive malignancies, CT has the advantage of easier accessibility, lower cost, and shorter scan times ( ; ; ). CT is also more sensitive for detecting early cortical destruction or sclerosis of laryngeal cartilage ( ; ). Nodal necrosis or cystic changes are imaging signs highly suggestive of lymph node metastasis and can be detected with ultrasound, CT, or MRI ( ). On CT, necrotic or cystic lymph nodes demonstrate central hypoattenuation with peripheral ring enhancement ( ; ).
Magnetic resonance imaging
MRI has several important advantages compared to CT, the most important being lack of ionizing radiation and superior soft tissue contrast. MRI is superior to CT in the evaluation of perineural tumor spread, for example along the pterygopalatine fossa ( ). Similarly, MRI is generally preferred over CT in tumors with suspected cranial nerve involvement ( ). While CT provides superior cortical bony details compared to MRI, early bone marrow replacement is often better delineated on MRI ( ; ; ).
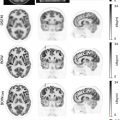
Certain dental hardware can cause imaging artifacts, obscuring adjacent anatomy, which can be particularly problematic in the assessment of oral cavity cancers. These artifacts can be minimized by adjusting scan parameters ( ; ; ). Temporary or permanent dental fillings or crowns can cause streak artifacts on CT, which are less apparent on MRI ( Fig. 3.1 ).
On MRI, like CT, nodal necrosis or cystic changes are highly suspicious for lymph node metastases ( ). A necrotic or cystic lymph node is often hyperintense in T2-weighted sequences (typical for a lymph node) with an irregular enhancing rim ( ). MRI is superior to CT in assessing retropharyngeal lymph node extension ( ; ). Given its superior soft tissue contrast, MRI is often the preferred modality in the preoperative evaluation for head and neck cancer ( ; ). Disadvantages of MRI include longer scan time, higher costs, and increased susceptibility to motion artifacts ( ).
Ultrasound
Compared to CT and MRI, ultrasound is widely accessible, portable, and relatively inexpensive. Unlike CT, ultrasound does not utilize ionizing radiation.
Ultrasound is especially useful when evaluating cervical nodal metastases, which are common in patients with head and neck cancer and are extremely important when considering prognosis and treatment options. Compared to CT, MRI, and PET/CT, ultrasound has the highest sensitivity for assessing malignant cervical lymph nodes. Greyscale sonographic features that help identify metastatic nodes include increased size, round shape, irregular or indistinct borders, and architectural changes, such as increased echogenicity, loss of a fatty hilum, necrosis, or calcification ( ; ). While CT and MRI are also useful for evaluating cervical lymph nodes, morphologic changes in small nodes (<5 mm) may be missed. For example, intranodal calcifications may be difficult to detect on MRI. In addition, color Doppler sonography can help identify metastatic lymph nodes by demonstrating peripheral or mixed (hilar and peripheral) vascularity, meanwhile normal or reactive nodes usually show hilar vascularity ( ).
Ultrasound also plays an essential role in image-guided tissue sampling ( ; ). Ultrasound-guided fine-needle aspiration with cytologic examination and/or core-needle biopsy can confirm node involvement with high specificity and fewer risks than excisional biopsy or surgery ( ; ). Disadvantages of ultrasound include its operator dependence and inability to evaluate deep spaces of the neck ( ).
Positron emission tomography/computed tomography
Positron emission tomography (PET) with [18F]-Fluorodeoxyglucose (FDG), most often paired with CT, is an essential tool for evaluating HNSCC ( ). FDG-PET/CT (subsequently abbreviated as PET/CT) has a high accuracy for initial staging, follow-up, and radiation treatment planning ( ; ). PET/CT can detect small tumors more effectively than either CT or MRI alone ( ). A small study of 35 patients demonstrated higher sensitivity of PET/CT compared to standalone MRI for diagnosing T1-T2 HNSCC due to FDG avidity of these tumors ( ). PET/CT can often be helpful when CT or MRI is unable to locate the primary site of disease ( ; ). PET/CT also identifies cervical lymph node involvement more accurately than CT or MRI ( ; ). For the evaluation of head and neck cancer without suspected cervical lymphadenopathy on physical exam, the sensitivity of PET/CT might be limited (50%–70%) but the specificity is over 80% ( ; ). For distant metastases, PET/CT is highly reliable, with sensitivity and specificity >90% ( ; ).
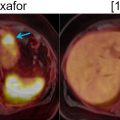
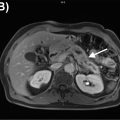
While PET/CT is useful for the staging and surveillance of head and neck cancer, it also has some important weaknesses. Exact tumor margins and involvement of surrounding structures is limited due to insufficient soft tissue contrast from CT ( ; ; ). In addition, superficial small mucosal lesions may not be identified and may be obscured by physiologic FDG uptake of pharyngeal lymphoid tissues, which could also be mistaken for malignancies ( Fig. 3.2 ) ( ; ).
PET/MRI in head and neck cancer
Commercial versions of whole-body PET/MRI have been available since 2010. first presented the feasibility of simultaneous PET/MRI acquisition with eight head and neck cancer patients ( ).
PET/MRI versus PET/CT
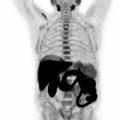
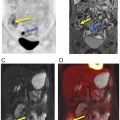
Compared to PET/CT, PET/MRI is more expensive and time-intensive. For head and neck cancers, the reliability of PET/MRI is at least equivalent and sometimes superior to PET/CT ( Fig. 3.3 ) ( ; ). The advantages of PET/MRI compared to PET/CT are mainly based on the simultaneous acquisition of PET and MRI and the superior soft tissue contrast of MRI compared to CT. In clinical practice, even when PET/CT is obtained, additional MRI scans may be needed to better delineate tumor margins and local and regional involvement ( ; ; ; ). In this regard, simultaneous PET/MRI could streamline the workup of these patients ( ). Lower radiation exposure in PET/MRI compared to PET/CT is another important advantage, particularly in the pediatric population ( ). Lesion conspicuity and diagnostic confidence are higher in PET/MRI than in PET/CT ( ; ; ).
PET/MRI protocol in head and neck cancer
PET/MRI acquisition protocols for head and neck cancer consist of small field of view PET/MRI scans of the head and neck and large field of view whole or partial body PET/MRI scans. The sequences used for the diagnostic MRI scan of the head and neck vary, depending on the institution’s preferences. MRI protocols typically include precontrast T1-weighted images without fat suppression, fat-suppressed T2-weighted images, fat-suppressed T1-weighted postcontrast sequences, and diffusion-weighted imaging (DWI). Various specific additional sequences may be added according to the individual clinical question, the location, and the extent of the tumor ( ). PET/MRI of the infrahyoid requires dedicated phased-array coils (head and neck or neurovascular). For nodal imaging, the field of view should include the area from the skull base to the level of the aortic arch ( ). Imaging coverage of the large field of view PET/MRI may be partial-body (skull base to mid-thighs) or whole-body (skull vertex to mid-thighs) ( ).
Evaluation of tumor extent
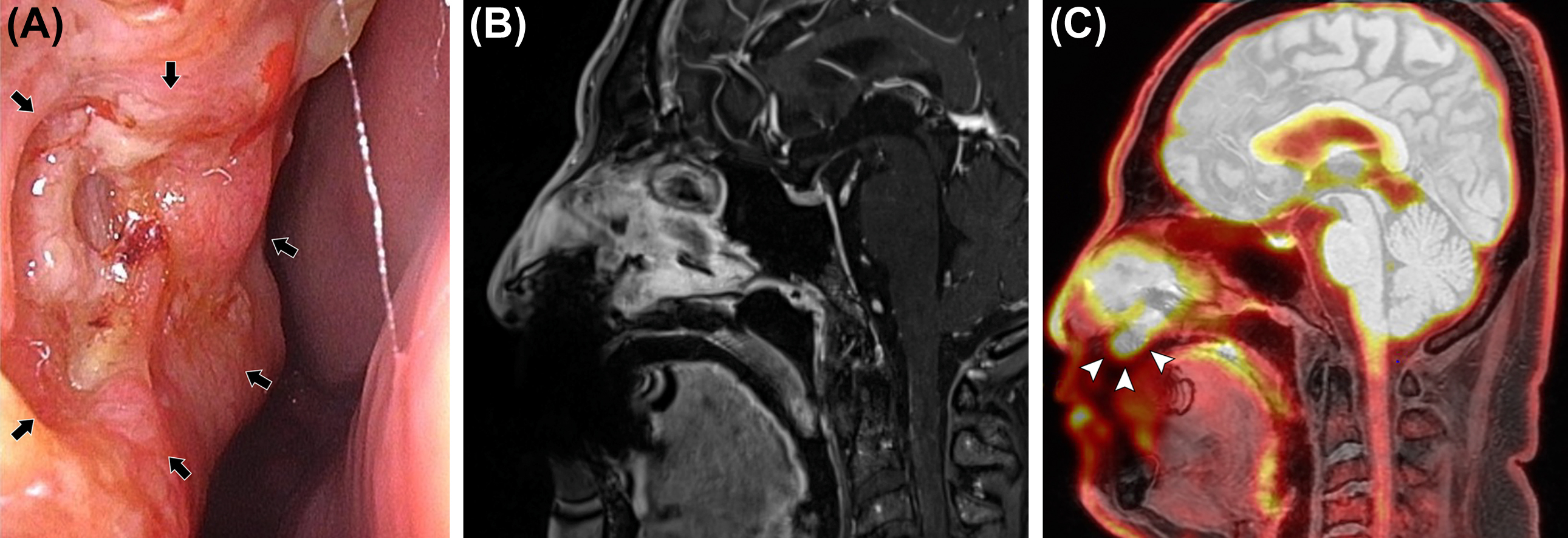
According to the eighth edition of the AJCC Cancer Staging Manual, T-staging is based on the size of the primary tumor and the extent of invasion of adjacent bones, nerves, muscles, and other soft tissues ( ). This staging predicts the possibility of surgical resection and defines the radiation field ( Fig. 3.4 ). The presence of perineural tumor spread is associated with poor prognosis and often changes the treatment plan, and thus preoperative detection is essential. MRI is superior at detecting perineural tumor spread because the contrast enhancement of nerve and perineural tissue on MRI is more prominent than on CT ( ; ; ). In more advanced stages of disease, CT indirectly depicts perineural tumor spread by demonstrating widening of the neural foramina or soft tissue enhancement ( ).
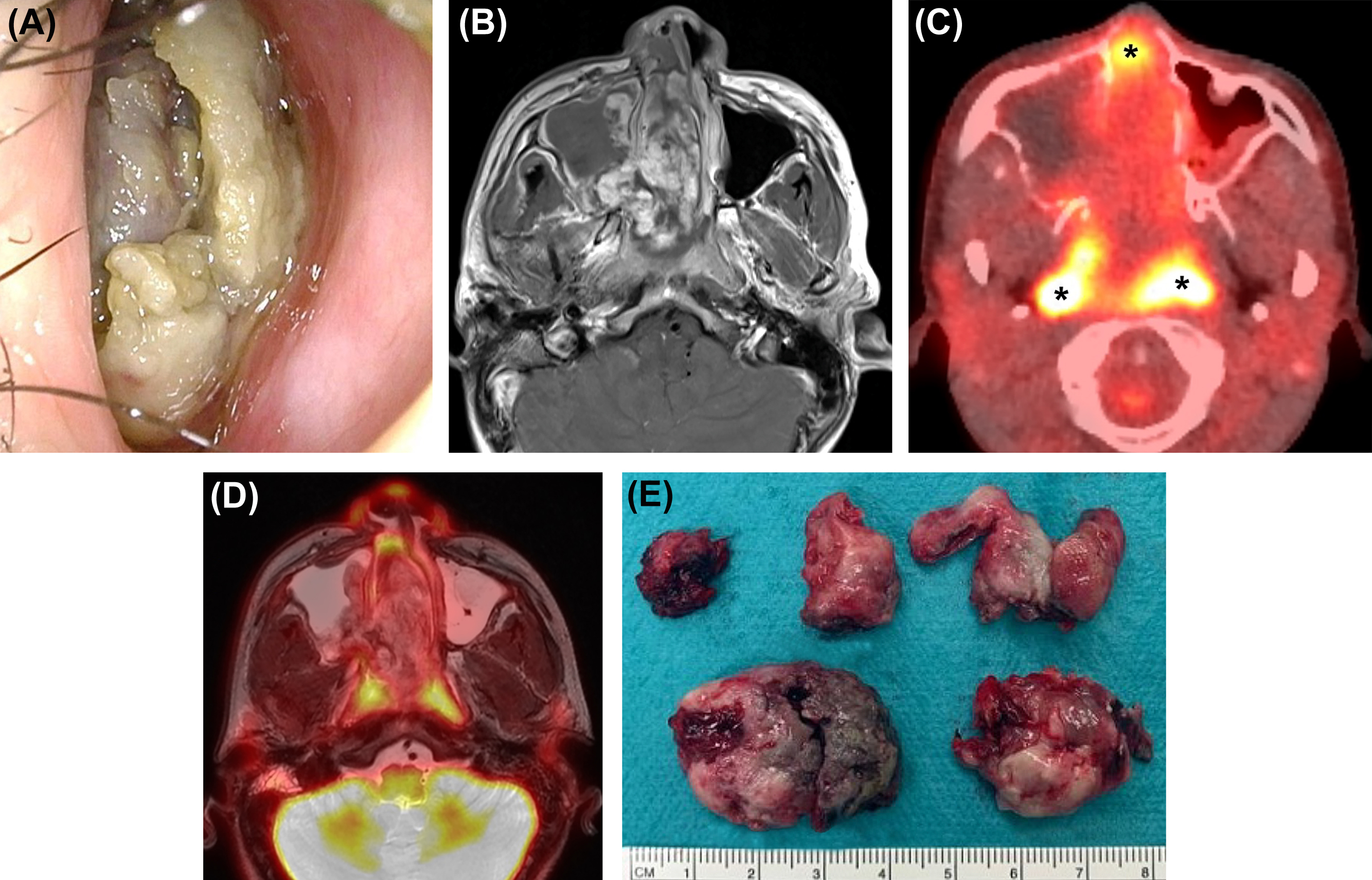
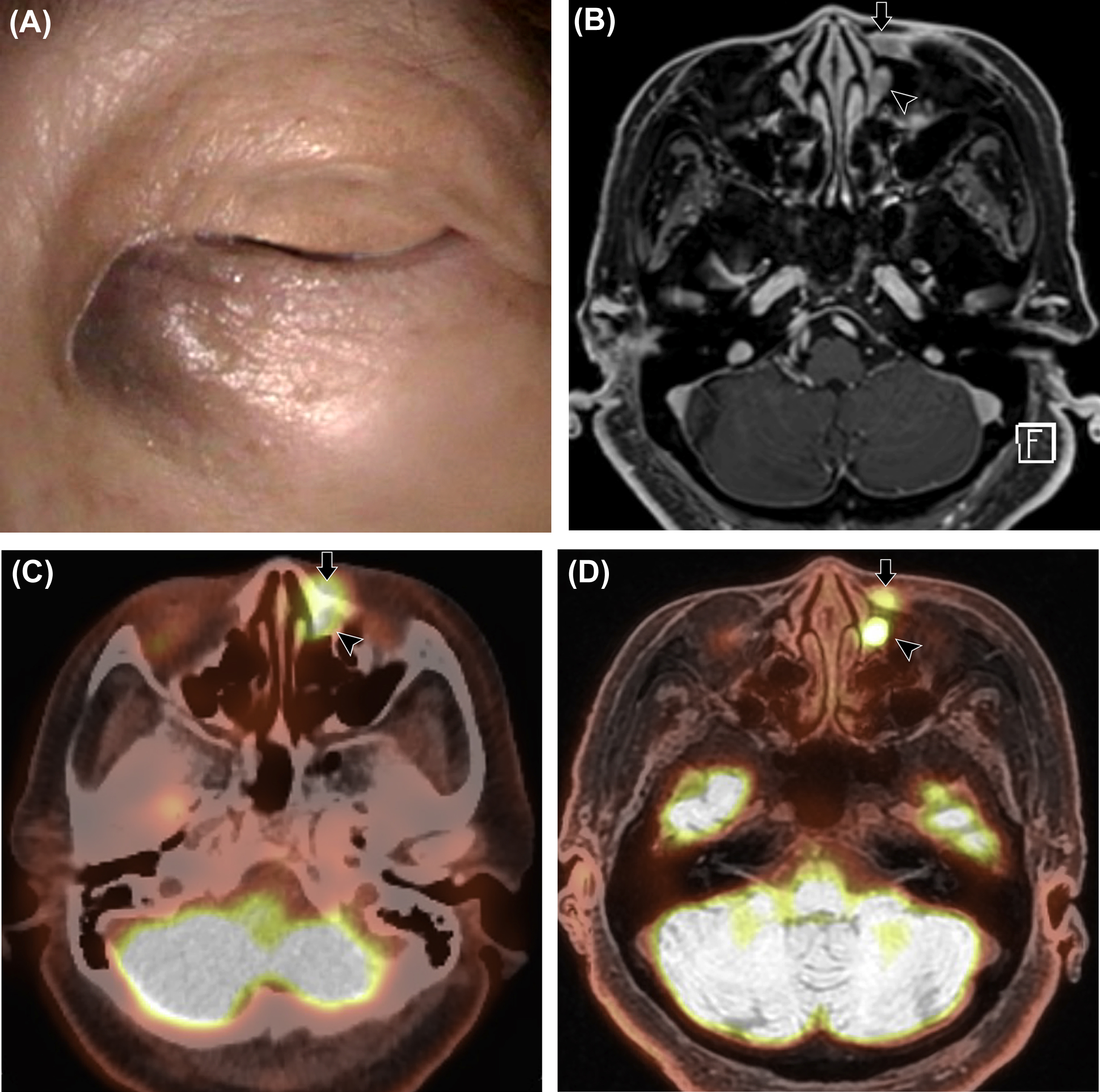
The nasopharynx is the most superior portion of the pharyngeal tube, bounded superiorly by the clivus and the floor of the sphenoid sinus, anteriorly by the nasal cavity or choanae, inferiorly by the soft palate, posteriorly by the prevertebral muscles and clivus, and laterally by the parapharyngeal spaces ( ). To determine the T-stage of nasopharyngeal cancer, evaluation for the presence of parapharyngeal, osseous, and intracranial extension is important. PET/MRI is beneficial for evaluating nasopharyngeal cancer that may spread through the parapharyngeal space into the masticator space or that may extend into the intracranial cavity via the foramen lacerum at the superior aspect of the nasopharynx ( ). It can also detect perineural tumor spread via the foramen ovale, which is an important pathway of nasopharyngeal cancer spread into the intracranial cavity. With PET/MRI, tumor involvement of the skull base, including the clivus, can be accurately evaluated even in the absence of cortical disruption. Several studies have reported that PET/MRI was more accurate than MRI and PET/CT for the staging of primary nasopharyngeal cancer ( ; ).
The oropharynx is bounded superiorly by the soft palate, inferiorly by the hyoid bone and vallecula, and anteriorly by circumvallate papillae along the posterior third of the tongue. T-stage of oropharyngeal cancer is determined by tumor size. In T4 disease, evaluation of tumor involvement of adjacent structures, including the larynx, tongue, masticator space, and bony structures, such as hard palate and mandible, is essential ( ).
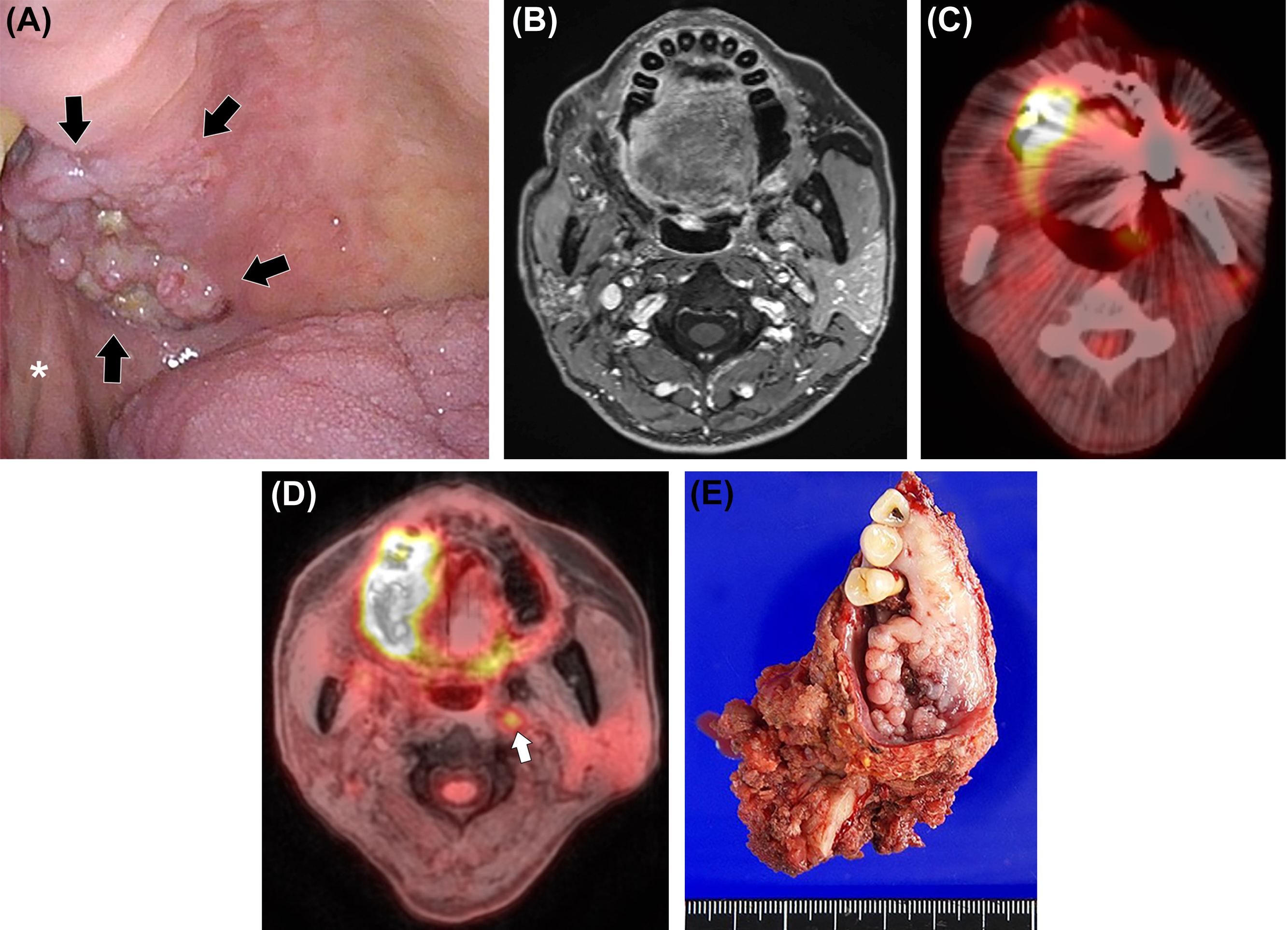
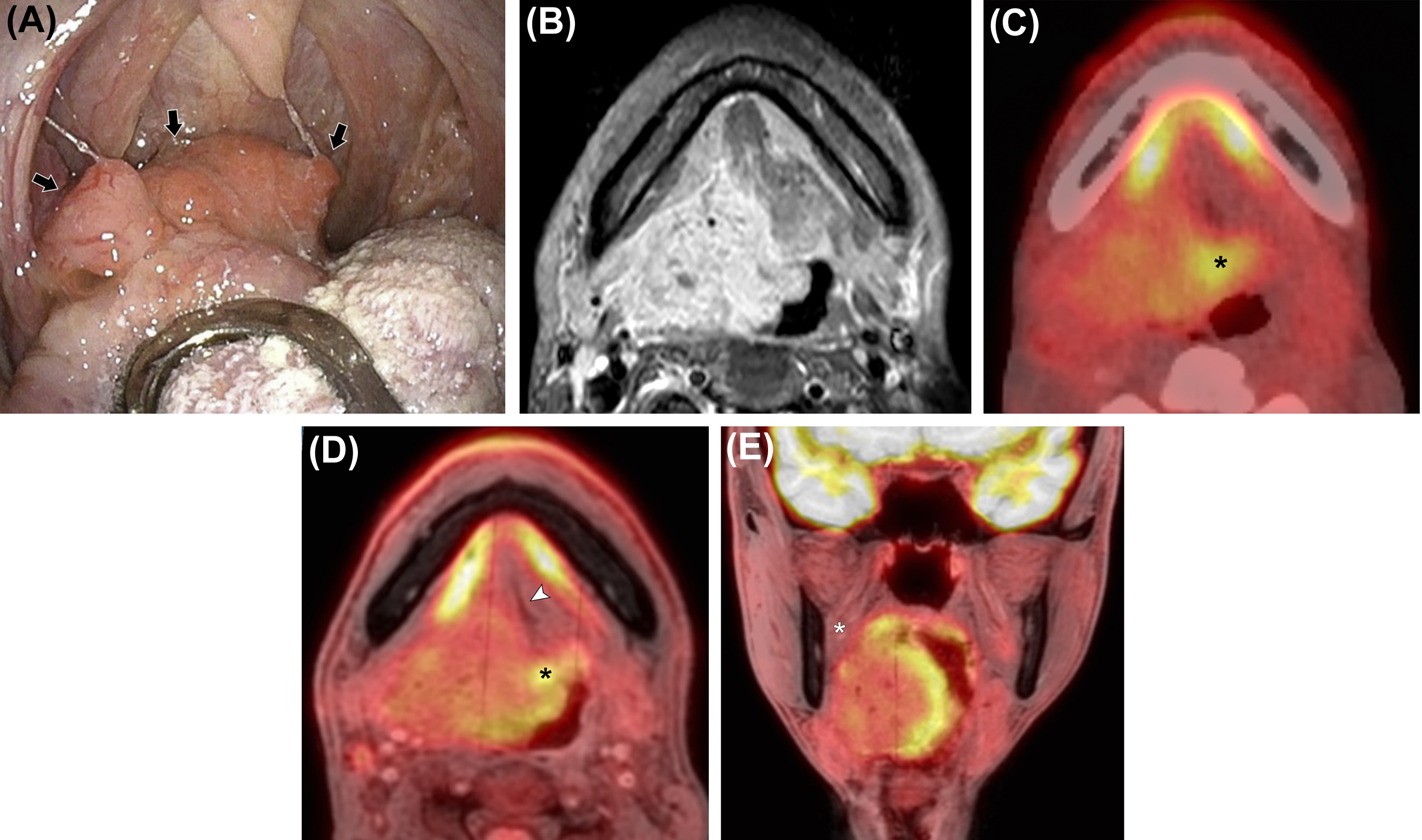
The oral cavity is bounded anteriorly by the lips, inferiorly by the mylohyoid muscle, the mandibular alveolar ridge, and the teeth, laterally by the gingivobuccal regions, superiorly by the maxillary alveolar ridge and teeth, and posteriorly by the tongue and tonsillar pillars ( ). To determine the T-stage of oral cavity cancer, correct evaluation of tumor size and depth is needed. Furthermore, in T4 stage disease, correct evaluation of the tumor involvement of adjacent bony structures such as mandible, maxilla, and pterygoid plate, and inferior alveolar nerve, in addition to perineural tumor spread is crucial ( ). PET/MRI is beneficial for the evaluation of the extent of oropharyngeal cancer and oral cavity cancer regarding tumor size and extension to the surrounding structures ( Fig. 3.5 ). This modality is also important for the evaluation of perineural tumor spread into the intracranial cavity via the pterygopalatine fossa from the oral cavity, especially in palatal cancer patients. As previously stated, an important advantage of PET/MRI over PET/CT for evaluation of the oropharynx and oral cavity is less pronounced artifacts from dental amalgams ( ). For lower T stage disease, beam hardening artifact on CT may hinder tumor detection and correct size measurement in oropharyngeal and oral cavity cancers. For T4 stage, it may preclude correct evaluation of tumor extent, such as invasion of the mandible. PET/MRI is more reliable for local invasion assessment and tumor size delineation in oral and oropharyngeal cancer compared with PET/CT, MRI, and CT ( ; ). PET/CT has the lowest confidence level, which may limit its use in the clinical setting ( ).
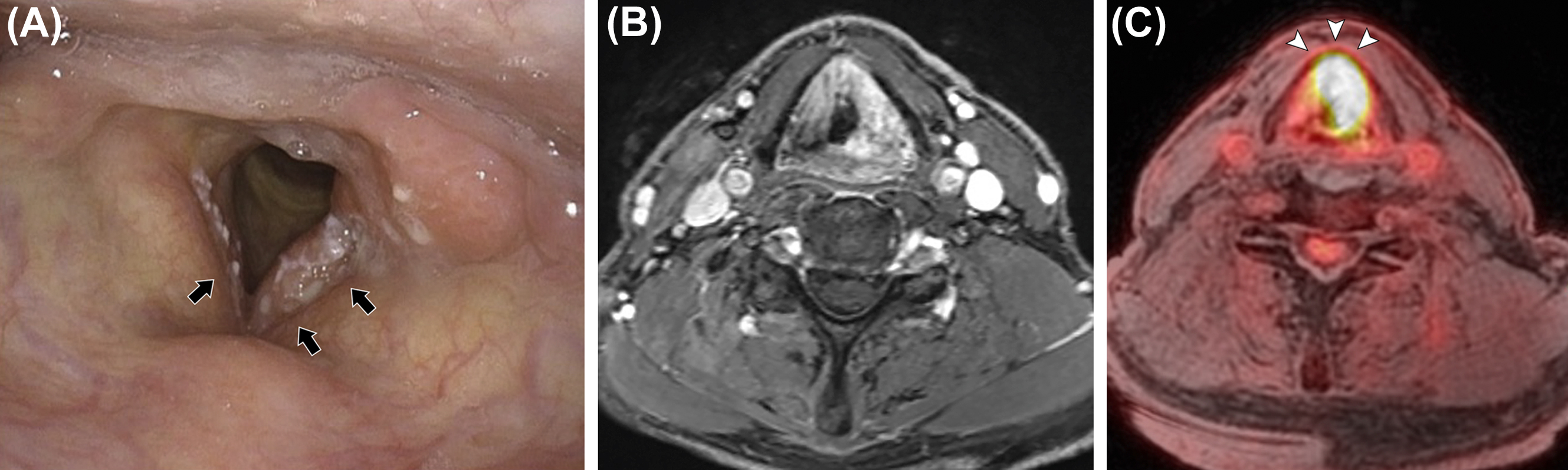
The hypopharynx and larynx are interrelated structures ( ). The larynx forms the anterior wall of the hypopharynx. The hypopharynx is bounded superiorly by the hyoid bone and inferiorly by the lower border of the cricoid cartilage. The hypopharynx is comprised of three subsites: the pyriform sinuses, the lateral and posterior hypopharyngeal walls, and the postcricoid region. The larynx is bounded superiorly by the free edge of the epiglottis and inferiorly by the lower border of the cricoid cartilage. The larynx is protected by an osseous and cartilaginous framework including the hyoid bone, the epiglottis, the thyroid cartilage, the cricoid cartilage, and the arytenoids ( ). The larynx is divided into supraglottis, glottis, and subglottis. The glottic larynx is composed of the laryngeal ventricles and true vocal folds. For lower T-stage disease, clear anatomic delineation of the tumor involvement is important ( Fig. 3.6 ). For T4 stage, evaluation of tumor involvement of the thyroid and cricoid cartilage and structures beyond the hypopharynx or larynx, such as prevertebral space, is crucial ( ). While the superior soft tissue contrast of PET/MRI may be advantageous in detecting tumor involvement of laryngeal cartilage and prevertebral space, evaluation may be limited by motion artifact from swallowing ( ). As such, it is unclear if PET/MRI outperforms PET/CT in laryngeal and hypopharyngeal cancer ( ; ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree