Introduction
Hybrid positron emission tomography (PET)/magnetic resonance imaging (MRI) is an emerging technology with promising applications in cancer evaluation ( ). The main advantage of these systems is that both anatomic and functional MRrI sequences are jointly acquired with metabolic PET data, resulting in high sensitivity for lesion detection and excellent soft tissue contrast resolution. Preliminary studies suggest that PET/MRI may improve whole-body assessment of soft tissues, facilitate characterization of small hepatic lesions, and provide a superior anatomic evaluation of the pelvis and abdomen than other imaging modalities, including PET/computed tomography (CT) ( ; ; ; ; ), with the added benefit of less radiation exposure ( ). Furthermore, PET/MRI may overcome some of the limitations of PET/CT, including decreased conspicuity of abnormal PET activity due to physiological uptake in background parenchyma, which may occur, for example, in the liver with 18 F-Fluorodeoxyglucose (FDG), or the uncinate process of the pancreas with somatostatin receptors (SSTR) agonists. Additionally, the inferior soft tissue contrast resolution intrinsic to CT can be particularly challenging in some cases, such as in detecting liver lesions in a background of hepatic steatosis.
Due to PET/MRI’s relative novelty, current National Comprehensive Cancer Network (NCCN) imaging guidelines for hepatobiliary and pancreatic cancers do not routinely include PET/MRI in the staging workup ( ; NCCN., NCCN Clinical Practice Guidelines in Oncology—Hepatobiliary Cancers Version 3.2021; ; ), except for neuroendocrine tumors. However, several published studies evaluated the impact of this technique in diagnosis, staging, and posttreatment assessment in hepatobiliary and pancreatic cancers ( ; ; ; ). For patients with hepatobiliary and pancreatic cancers, accurate diagnosis and staging, as well as a precise anatomic map for treatment planning, are essential to disease treatment and prognosis. Throughout the chapter, we describe how PET/MRI may improve the diagnosis, staging, and post-treatment assessment of such tumors in light of the currently available data.
Protocols
Sequences
Our hepatobiliary/pancreatic protocol consists of a noncontrast-enhanced whole-body PET/MRI acquisition and a dedicated contrast-enhanced upper abdominal PET/MRI acquisition. The incubation time for either FDG or SSTR agonists ranges between 45 and 60 min.
The whole-body noncontrast-enhanced PET/MRI is acquired from the mid-thighs to the base of the neck and consists of a two-point Dixon sequence for attenuation correction, an axial T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE/SSFSE), and an axial simultaneous multislice (SMS) diffusion-weighted imaging (DWI) sequence. SMS-DWI, when compared to standard single-shot echo-planar imaging (SSEPI) DWI, decreases acquisition time by roughly 50% while maintaining or even improving sensitivity, according to our initial unpublished data.
The dedicated upper abdominal protocol includes the following sequences: precontrast coronal T2-weighted HASTE, axial T2-weighted fat-suppressed fast spin-echo (FSE), axial T1-weighted Dixon, and finally dynamic contrasted-enhanced (CE) sequences. Most commonly, extracellular gadolinium-based contrasts are used. Alternatively, hepatocyte-specific contrast agents, such as gadoxetate, may be used for the detection and assessment of focal liver lesions. Magnetic resonance cholangiopancreatography (MRCP) sequences may be added in the case of biliary or pancreatic cancers. The entire examination lasts 45–60 min.
Radiopharmaceuticals
FDG is the workhorse radiopharmaceutical in most hepatobiliary and pancreatic imaging. When using FDG, we recommend ≥6 h fasting before PET/MRI scanning to ensure adequate glucose/insulin homeostasis, with a target blood glucose level of less than 200 mg/dL ( ). In patients with diabetes or poorly controlled blood glucose levels, due to severe pancreatic pathologies or post pancreatectomy, short-acting insulin may be administered to bring the blood glucose level to a more acceptable value before injecting FDG ( ; ). Ideally, the interval between insulin injection and FDG administration should be at least 1 h ( ). In insulin-dependent diabetic patients, we recommend fasting for ≥4 h.
While higher blood glucose levels may not significantly impact the physiologic FDG uptake in organs other than the brain ( ; ), tumor uptake may be altered, thus rendering semiquantitative parameters such as the standardized uptake value (SUV) both unreliable and unreproducible. A possible workaround in these instances is a correction of SUV to the blood glucose level ( ).
FAPI is a very promising radiopharmaceutical to assess cholangiocarcinomas and hepatocellular carcinomas (HCC), with reported sensitivities of 85.7%–100% in mixed cholangiocarcinoma/HCCs populations. Moreover, in primary hepatic malignancies, the reported tumor to background ratio is way higher with FAPI (15.18) than with FDG (2.08) ( ; ; ; ).
In well-differentiated neuroendocrine tumors, SSTR agonists radiopharmaceuticals are used, such as 68 Ga-DOTATOC or 68 Ga-DOTATATE ( ; ). On the other hand, in the case of poorly differentiated neuroendocrine tumors, with low expression of SSRT but high mitotic activity and sheer glycolytic metabolism, FDG is a more ideal option.
For HCC, 18 F or 11 C-Coline, and 68 Ga-PSMA are options, potentially better than FDG ( ; ). 68 Ga-PSMA PET, predominantly used in prostate cancer imaging, is also taken up by benign entities and other malignancies, including HCC, as it binds to neovasculature ( ; ; ; ). 11 C-labeled acetate ( 11 C-ACT) and 18 F-Fluorocholine (CHOL) have also been used to evaluate HCC.
Primary hepatic malignancies
Hepatocellular carcinoma
HCC is the most common primary liver cancer and the third cause of cancer-related deaths worldwide ( ; ; ) HCC is increasing in incidence, with risk factors including cirrhosis and chronic viral hepatitis ( ; ). Early imaging diagnosis of HCC is critical, as there is curative potential with surgical resection or liver transplantation in early-stage tumors.
Current standard of care imaging
When evaluating hepatic lesions, MRI has high accuracy in the identification and characterization of HCC and better delineates intrahepatic lesions when compared to CT or PET/CT, commonly obviating the need for tissue confirmation. FDG-PET alone is not typically used in the evaluation of HCC, due to its low overall sensitivity of approximately 64% ( ; ). This is in part explained by the stronger enzymatic activity of well-differentiated HCC, resulting in less FDG uptake, compared to possibly high and heterogeneous uptake of background liver.
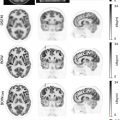
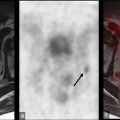
However, since tissue sampling is uncommonly needed for diagnosis, the biological behavior of these tumors is often unknown; this is a potential area where FDG-PET may provide additional information. While well-differentiated HCC demonstrates less FDG uptake, poorly differentiated HCC has weaker enzymatic activity ( ), which leads to increased FDG uptake in up to 40% of cases Fig. 11.1 .

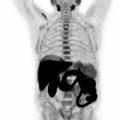
Therefore, it may be possible to predict the degree of differentiation of HCC based on its FDG uptake, providing insight into tumor biology and prognosis. Poorly differentiated HCC also tends to recur and metastasize, making whole-body imaging with FDG-PET more useful ( ), as FDG-PET has a high sensitivity for detecting distant extrahepatic metastases ( ; ). FDG-PET/CT can also differentiate neoplastic from bland portal venous thrombosis, which can also affect prognosis, therapeutic approach, and eligibility for liver transplantation ( Fig. 11.2 ). Therefore, FDG-PET can be considered for initial HCC staging of candidates for hepatic resection (HR) or transplantation ( ).

In terms of prognostic implication, FDG uptake can also predict therapeutic response and provide information about the risk of recurrence after surgery or transplant ( ; ). Moreover, the tumor-to-liver FDG uptake ratio can predict microvascular invasion ( ). Hypermetabolism on PET has been related to early recurrence of HCC after orthotopic liver transplant, and, when combined with elevated alpha-fetoprotein levels, can provide even greater predictive value.
Regarding post-treatment evaluation, FDG-PET has been used to evaluate early response after transarterial chemoembolization (TACE), by quantifying differences in SUV of the HCC before and after TACE ( ; ; ). Other studies demonstrated increased sensitivity of PET/CT over CT or MRI alone for detecting recurrence after radiofrequency ablation ( ).
In recent studies, 68 Ga-PSMA PET has demonstrated high sensitivity in detecting primary HCC (97%, comparable to CT) and distant metastases (100%, outperforming CT) ( ). PSMA-PET led to management changes in 33% of the patients. A separate study showed PSMA uptake in all 15 patients with HCC, without significant differences in SUV measurements or tumor-to-liver ratios between untreated and previously treated patients ( ). Moreover, in the setting of HCC, PSMA-PET detects more lesions than FDG-PET/CT ( ). Unlike CT and MRI, PSMA-PET allows for adequate evaluation even if the background parenchyma has architectural and anatomic abnormalities ( ).
11 C- and 18 F-Fluorocholine (CHOL) are other promising radiopharmaceuticals for the evaluation of HCC, with improved tumor-to-background contrast ( ) and high sensitivity for intrahepatic HCC (89%) and extrahepatic metastases (100%) ( ; ; ; ).
FAPI is an extremely appealing radiopharmaceutical for evaluating HCC; according to preliminary studies, its uptake is inversely correlated to HCC grading and directly correlated to HCC size. FAPI tends to outperform FDG in local and whole body staging. Reported sensitivities for detecting intrahepatic HCC with FAPI and FDG were 68.8% versus 18.8% in tumors <2 cm, 100% versus 81.8% in tumors 2–5 cm, and 100% for both in HCC >5 cm ( ).
PET/MRI in hepatocellular carcinoma
FDG-PET/MRI may improve the sensitivity and specificity of HCC whole-body staging when compared to chest, abdomen, and pelvis CT or abdominal MRI. As morphologic changes can lag behind metabolic changes in soft tissue, FDG-PET/MRI may be helpful in the detection of metastatic disease, particularly in otherwise occult osseous and nonregional lymph node metastases. This can result in management changes in up to 10% of patients, especially those with locally advanced HCC ( ). Additionally, FDG-PET in combination with multiparametric contrast-enhanced MRI could improve the evaluation of residual or recurrent HCC. In patients undergoing 90 Y radioembolization, FDG-PET/MRI may help establish target lesion dosimetry, which could allow for prediction of treatment response before radiographic evidence ( ).
Cholangiocarcinoma
Cholangiocarcinoma is the second most common primary hepatic neoplasm and bears a poor 5-year survival rate of 10% ( ). The worldwide incidence of cholangiocarcinoma, particularly intrahepatic cholangiocarcinoma (ICC), has increased globally over the past few decades and continues to increase ( ). Cholangiocarcinoma can arise anywhere along the biliary tree and can be categorized as intrahepatic versus extrahepatic, with extrahepatic disease further subdivided into distal and perihilar. Each subtype has distinct epidemiology, biology, prognosis, and clinical management, which are strongly influenced by the stage at presentation.
Current standard of care imaging
The only curative treatment for patients with cholangiocarcinoma is surgical resection, which makes accurate staging and patient selection crucial. Surgical resection with negative margins is the most important factor in achieving long-term survival, and several recent innovative approaches have increased the number of patients that reach this goal. A multimodality imaging approach is necessary when staging patients with cholangiocarcinoma, including contrast-enhanced CT, MRI with MRCP, and PET to assess tumor extent, relationship with adjacent structures, regional lymph node involvement, and distant metastases ( ; ; ; ; ; ).
NCCN guidelines recommend CT or MRI for diagnosis and staging of cholangiocarcinoma ( ; NCCN., NCCN Clinical Practice Guidelines in Oncology—Hepatobiliary Cancers Version 3.2021.; ). In patients with hilar cholangiocarcinoma, MRCP and endoscopic retrograde cholangiopancreatography are primarily used for evaluating the extent of ductal involvement, with moderate to high accuracy ( ). Both CT and MRI have intrinsic limitations in detecting distant metastases, intrahepatic metastases smaller than 10 mm, and peritoneal metastases. Morphologic imaging is also often inadequate in assessing lymph node involvement. Moreover, the evaluation of microscopic peritoneal disease is even more challenging. Hence, in cases of potentially resectable ICC by imaging, a staging laparoscopy should be considered according to NCCN guidelines, as up to 36% of the cases considered resectable on CT or MRI will reveal unresectable advanced disease at laparoscopy ( ; ).
FDG-PET may be useful in detecting the primary tumor and metastases, and for differentiating between benign and malignant biliary strictures. This may explain the management changes that occur in up to 15.9% of patients with potentially resectable cholangiocarcinoma who undergo FDG-PET/CT ( ). Despite the avidity of FDG uptake by the epithelial component of cholangiocarcinomas, the abundant fibrous stroma, with low glycolytic metabolism, may decrease the overall FDG avidity. As a result, the sensitivity of FDG-PET inversely correlates with the abundance of tumor stroma and is higher, for example, in papillary ductal cholangiocarcinoma than in periductal infiltrating tumors ( ).
On the other hand, FAPI does not suffer of these limitations and demonstrates remarkably high uptake with prominent tumor to background ratio in cholangiocarcinomas ( ; ; ).
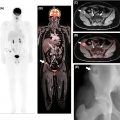
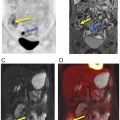
Given its low soft tissue resolution, PET/CT may be of limited use to evaluate vascular involvement, especially when the CT component is acquired with a low radiation dose for attenuation correction purposes only. Moreover, in the case of extrahepatic cholangiocarcinomas, the insertion of biliary stents can induce local inflammatory changes and wall thickening, rendering the distinction between inflammation and neoplasia difficult. Conversely, false-negative results may occur in small lesions and those with a predominance of fibrous stroma or high mucin content ( ) Fig. 11.3 .

PET/MRI in cholangiocarcinoma
PET/MRI may overcome some inherent limitations of PET/CT by leveraging the simultaneous acquisition of PET along with the superior depiction of the biliary tree through T2- weighted sequences, including 3D MRCP, and the evaluation of the bile duct morphology and vascular involvement using contrast-enhanced sequences ( Fig. 11.4 ). This approach also facilitates the detection and characterization of small intrahepatic metastases, even in cases with concurrent cholangitis or postradiation changes. In one study, FDG-PET/MRI impacted clinical management in 29.7% of the patients with untreated mass-forming ICC, allowing a more precise assessment of the intrahepatic extent of disease as well as detecting or ruling out distant metastases ( ). However, the impact of PET/MRI compared to PET/CT is likely less significant in patients presenting with already widely metastatic disease at the time of diagnosis.

Pancreatic cancer
Pancreatic adenocarcinoma
The incidence of pancreatic cancer continues to rise. Pancreatic ductal adenocarcinoma (PDAC) is the prevailing histologic subtype, accounting for over 90% of cases, and carries a dismal prognosis with a 5-year overall survival rate of approximately 8% ( ; ), due to few successful treatment options and frequent late presentation. Surgery is considered the only curative treatment, but most patients are not eligible because of advanced disease at the time of diagnosis. Accurate diagnosis, staging, and surveillance are critical for these patients, with imaging playing a central role.
Current standard of care imaging
Currently, CE-CT is the preferred staging method, followed by CE-MRI. The accuracy of PET/CT in detecting PDAC is equivalent or superior to CE-CT or CE-MRI, and FDG-PET/CT might affect management. However, it is not universally used as a standard of care ( ; ). Hyperglycemia or overt diabetes, which can be present with PDAC, pose specific challenges with the use of FDG. In fact, hyperglycemia decreases FDG uptake in primary pancreatic tumors and could result in a falsely negative scan ( ). In our practice, when needed, we administer short-acting insulin, at least 60 min before FDG injection, to achieve blood glucose levels under 200 mg/dL.
FAPI, whose uptake is not influenced by glycemic level, and which targets cancer associated fibroblasts that are abundant in the desmoplasic reaction typical of pancreatic adenocarcinomas, has shown high uptake in primary and metastatic PDAC, and will likely play a major role in diagnosing and staging this cancer ( ).
PET/MRI in pancreatic adenocarcinoma
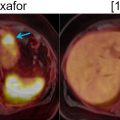
In preliminary studies, FDG-PET/MRI was superior or equal to FDG-PET/CT and CE-CT in pancreatic tumor characterization, diagnosis, staging, and preoperative assessment of resectability ( ; ) ( Fig. 11.5 ). In a dedicated study, FDG-PET/MRI led to management changes in 49% of PDAC patients compared to standard of care imaging (CT, PET/CT, and MRI) ( ). In PDAC, FDG-PET/ MRI has the potential to provide incremental diagnostic accuracy over FDG-PET/CT and can be complementary to conventional imaging for metastatic disease evaluation. Additionally, FDG-PET/MRI has a high specificity in detecting locoregional lymph nodes ( ).

CE FDG-PET/MRI may also improve detection of peritoneal metastases. In a prospective study that enrolled a mixed cancer patient population, including pancreatic adenocarcinomas, CE FDG-PET/MRI, outperformed conventional imaging, including PET/CT, in assessing for peritoneal disease, with sensitivity of 97% versus 54%, and negative predictive values of 99% versus 88%. These figures were the result of simultaneously acquired PET and MR data and combined assessment of increased, even if mild, FDG uptake with enhancement of the peritoneum, using the pleura as internal reference (Catalano’s pleuroperitoneal sign) ( ).
FDG-PET/MRI can be used for evaluating response to treatment in borderline resectable PDAC. Tumor size changes according to RECIST (Response Evaluation Criteria in Solid Tumors), measured on CT, are known to underestimate the effectiveness of neoadjuvant therapies. PET/MRI may be useful by combining functional and metabolic parameters such as SUV and the apparent diffusion coefficient (ADC), which are independent surrogate biomarkers for tumor response assessment ( ; ). In another study, FDG-PET/MRI biomarkers, namely, high ADC min (minimum apparent diffusion coefficient) and low total lesion glycolysis correlated with better overall survival in patients with PDAC ( ).
In the evaluation for recurrent disease after surgical resection, the excellent spatial colocalization enabled by PET/MRI, combined with superior soft tissue layout and high contrast-to-noise ratio, may improve the detection of recurrence in patients with complex postsurgical anatomy or those with equivocal findings on CT ( Fig. 11.6 ) However, considering cost-effectiveness, it is unlikely that PET/MRI will replace CT in the diagnosis and initial staging of PDAC in the near future. Nevertheless, it may have a role in borderline resectable PDAC and suspected recurrence with negative or indeterminate CT, MRI, or PET/CT findings.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree