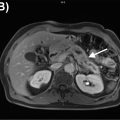
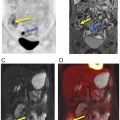
Introduction
Blood malignancies account for approximately 10% of newly diagnosed malignant tumors in the United States. While a large number of different blood cancer entities exist, three main groups are generally recognized:
- 1.
Lymphoma, which is responsible for almost 50% of new cases
- 2.
Leukemia which accounts for 35% of new cases
- 3.
Myeloma, which accounts for 18% of new cases.
Due to their unique biological and pathophysiological features, these blood cancer groups require different approaches not only to clinical management including treatment, but also diagnostic workup, which today relies heavily on diagnostic imaging. While imaging plays only a limited role in leukemia—at least those leukemia variants that do not have a lymphoma-like course, such as chronic lymphocytic leukemia (CLL)—it is of great importance in lymphoma and myeloma. Hybrid imaging, which brings together techniques from radiology and nuclear medicine, is particularly relevant in that regard, and steadily increasing terms of application.
In this chapter, we will evaluate the clinical and histological background and the current state-of-the-art imaging of two of the main blood cancer groups—lymphoma and myeloma—with a focus on positron emission tomography (PET)/ magnetic resonance imaging (MRI) as the latest hybrid imaging technique to be introduced into clinical practice. Similar to other cancers, PET/MRI is not, or at least not yet, a standard test in these blood cancers. Nevertheless, several applications exist in which PET/MRI may be superior, or at least, noninferior to currently used imaging tests. The chapter will also focus on recent developments in terms of biomarkers that are, or at least might be, candidates for future application in routine clinical practice. Another focus of this chapter will be on treatment response assessment, for which imaging is already heavily utilized, but for which improvements are still possible.
PET/MRI protocols overview
Contrary to PET/MRI protocols for other cancers, protocols for hemato-oncological malignancies are usually uniform across the entire body. This is because these cancers are considered systemic diseases that—with the exception of a few lymphoma types—do not have a distinct site of origin or primary site. Typical PET/MRI protocols include only a small number of unenhanced pulse sequences to capture anatomy, as well as diffusion-weighted imaging (DWI) MRI sequences obtained in free-breathing. DWI has shown high sensitivity for blood cancers detection due to their high cellularity. Such short protocols also enable PET/MRI scans to be performed in a reasonable amount of time (i.e., 30–40 min). Beside the axial plane, which is the most important for MRI, at least one additional imaging plane should be included. In lymphomas, the coronal plane is usually preferred, due to the need for determining splenomegaly; whereas in myeloma, sagittal spine sequences add relevant information, given that fractures are common. FDG is the current PET tracer of choice in blood cancers, in particular lymphomas, and is obtained according to standard PET acquisition guidelines. As explained in more detail below, delayed time point imaging may, however, sometimes be useful in certain lymphomas.
Lymphoma
Histology and clinical background
The current 2016 WHO classification of lymphoid tumors recognizes a wide variety of lymphoma subtypes ( ), detailed knowledge of which may not be required for the radiologist or nuclear medicine physician in his clinical practice. However, knowledge of the most common lymphoma subtypes is relevant and helpful for correct imaging interpretation and also, for determining the most appropriate imaging test.
Hodgkin lymphoma, which accounts for 9%–10% of all lymphomas, typically occurs in young adults, although there is a second peak in adulthood. Classical Hodgkin lymphoma is found in >90% of patients and has as its histological tell-tale sign the so-called Reed-Sternberg cells (which originates from B lymphocytes), with a marked surrounding immune infiltrate that leads to the actual lymphadenopathy ( )—Reed-Sternberg cells themselves constitute less than 1% of the tumor mass. With 5%–10%, nodular lymphocyte-predominant Hodgkin lymphoma is the second variant of Hodgkin lymphoma and usually has a different, more slowly progressing clinical course ( ).
Neck and chest lymph nodes are the most common sites of Hodgkin lymphoma, with the spread from one lymph node station to the neighboring one as the expected route of progression. Nevertheless, extranodal spread, particularly to the lungs, spleen, liver, kidneys, and bone marrow is however also frequently observed. In larger nodal lymphoma bulks, the criterion for which is a 10 cm maximum diameter according to the Ann Arbor classification ( ), cystic components are frequently seen in the mediastinum (probably of thymic origin) ( )—and often mistaken for necrosis. Hodgkin lymphoma generally has an excellent prognosis, at least when compared to many other non-Hodgkin lymphomas, with cure rates of 85%–90% using standard chemotherapy ( ), or less frequently immunochemotherapy regimens.
Non-Hodgkin lymphomas on the other hand are a far more heterogeneous and, in terms of biological characteristics, diverse group of lymphoid neoplasms. Over 60 entities are recognized in the WHO classification ( ), the most common ones being: diffuse large B cell lymphoma with 30%–35% of cases; follicular lymphoma with 25% of cases; marginal zone lymphoma (MZL) with 10%–12% of cases; CLL/small-cell lymphocytic lymphoma (CLL/SLL), 7%–8% of cases; and mantle cell lymphoma (MCL), with up to 7% of cases. Based on the REAL classification ( ), DLBCL represents the most common aggressive, fast-growing NHL subtype, whereas follicular lymphoma is the most common indolent subtype and can, similarly to MZL and CLL/SLL, transform into an aggressive histology over time. Prognosis varies heavily within the NHL group, with MCL, which can have an aggressive or indolent course, generally having the poorest prognosis, with a 5-year survival rate of just about 50% ( ). Treatment approaches also vary considerably, ranging from antibiotic treatment or local radiation therapy in the case of extranodal mucosa-associated lymphoid tissue MZL (MALT lymphoma) to immunotherapy and/or chemotherapy ( ). More cutting-edge treatments, for instance with targeted therapies such as Bruton’s tyrosine kinase inhibitor ibrutinib, or anti-PD-1 or -PD-L1 checkpoint inhibitors, are also increasingly used both in indolent and aggressive lymphoma subtypes ( ; ; ).
Current imaging state of the art
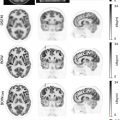
According to the current Lugano classification of the International Conference on Malignant Lymphomas (ICML), the two preferred imaging techniques for assessment of disease extent, disease staging and treatment response assessments are FDG-PET/CT and contrast-enhanced CT (CE-CT) ( ; ). Since the vast majority of lymphoma subtypes show an elevated glucose metabolism, FDG-PET/CT is generally the preferred test in lymphomas ( ; ). FDG uptake is considerably higher in aggressive NHL as well as Hodgkin lymphoma, with typical maximum standardized uptake values (SUV max ) between 10 and 30. Other, more indolent subtypes, including follicular lymphoma, show moderate, but still elevated FDG uptake relative to the background. However, some very slowly growing lymphomas such as the MZL group (which consists of nodular, splenic, and MALT variants), CLL/SLL, lymphoplasmacytic lymphoma (macroglobulinemia of Waldenström), and also cutaneous T cell lymphomas show a variable, frequently very low FDG uptake ( Fig. 9.1 ) ( ; ). It is for the latter group that PET/CT is not recommended by the ICML, and thus, CT remains the standard test.

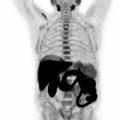
Notably, MRI is currently only officially recommended for the assessment of CNS lymphoma ( ), because the CNS shows a high physiologic FDG uptake that leads to a markedly reduced tumor to background contrast. This is surprising given that MRI also offers superior soft-tissue evaluation in many anatomic sites such as the head and neck ( Fig. 9.2 ) and that there is an increasing body of evidence that supports the use of whole-body MRI as a possible alternative to PET/CT. Whole-body MRI appears to be only slightly to moderately inferior to FDG-PET/CT ( ; ; ; ), especially when DWI sequences are included in the MRI protocol. This is because DWI can indirectly capture cell density—which is generally high in lymphomas—and, by using apparent diffusion coefficients based on DWI, quantification of treatment response in terms of reduction in cellularity is possible ( ). Nevertheless, a limitation of whole-body MRI, and DWI in particular, is its sensitivity to motion artifacts, especially in the mediastinum (due to breathing and cardiac motion) and the lower neck, which are two common sites of lymphoma involvement. Given its lack of ionizing radiation, however, MRI may be considered an alternative technique in children and young adults, who might require lifelong imaging follow-up. Also, patients undergoing so-called watchful waiting (active surveillance), which is a frequently employed management strategy in indolent lymphomas, represents another possible application of whole-body MRI ( ).

Lymphoma staging
The Lugano classification includes a slightly modified version of the well-known Ann Arbor staging classification. The main concepts remain unchanged: there are four stages of lymphoma, the main difference between stages I-II and stage III being whether the disease is limited to one side of the diaphragm, or whether both sides of the diaphragm are involved. Stages I-III may not only include nodal involvement (lymphadenopathy), but may also include extranodal involvement (i.e., involvement of solid organs, soft tissues, and bone), as long as only a single extranodal site with only a single lesion is present according to the Ann Arbor system, or if extranodal disease is limited/localized or contiguous from a nodal lymphoma manifestation ( ). Once extranodal involvement is multifocal or diffuse, or in the case of multiorgan involvement, the criteria for stage IV disease are fulfilled. This is particularly important with regard to bone involvement, which represents a major challenge for imaging ( ).
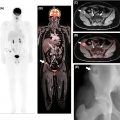
Bone involvement can manifest as focal or multifocal disease, or as diffuse marrow involvement, a pattern that is more frequently observed in indolent lymphomas. Imaging studies have so far been unsatisfactory in terms of assessment of bone marrow involvement. According to Lugano guidelines, PET/CT can only replace bone marrow biopsy (BMB) in patients with Hodgkin lymphoma, and also in patients with DLBCL and unequivocal foci of increased FDG uptake (with, or—as is commonly the case—without morphological correlate on CT) ( ). All other patients must undergo BMB to exclude marrow involvement. Whether BMB is actually performed depends on the clinical situation, the institutional standard of care, and also on the lymphoma subtype, and the likelihood of marrow involvement. For instance, bone marrow involvement in MALT lymphoma is relatively rare, whereas in MCL it occurs in 55%–90% of cases at the time of diagnosis ( ). Here, novel imaging tests are clearly needed, given that the bone marrow is the only extranodal site that currently requires an invasive test, rather than noninvasive imaging, to assess the presence of disease in the vast majority of NHL subtypes. Notably, FDG-PET may be false negative or difficult to interpret early after application of chemotherapy or granulocyte colony stimulating factors, when a diffuse increased uptake is a common finding ( Fig. 9.3 ).

Another organ that requires special mention is the spleen. In terms of staging, the Ann Arbor system evaluated the spleen separately, and involvement of the spleen was denoted by adding the letter “S” to the stage (e.g., stage IIS). In the Lugano system this strategy has been modified, and similar to the Waldeyer ring, the spleen is now regarded as a lymphatic (=nodal) tissue ( ; ). This means that, due to the infradiaphragmatic location of the spleen, involvement can directly influence the stage if it occurs in addition to supradiaphragmatic nodal involvement—this would then be regarded as stage III. The spleen is considered involved if there is diffuse uptake higher than the liver background (unless PET is performed shortly following treatment, in which case it might be physiological) if there are focal FDG avid lesions; or, in case of lack of FDG-PET information, if there are suspicious focal lesions on CT, or if there is enlargement of the spleen with a vertical diameter of more than 13 cm, at least in the absence of other factors to explain this splenomegaly, such as portal venous hypertension, or immune-related events. Note that oblique spleen measurements in the axial or coronal planes are not permitted; a straight craniocaudal line on coronal CT (or MRI, or coronal PET reformations) is used for assessment of splenomegaly.
Treatment response assessment in lymphoma
Treatment response assessment not only plays a major role in routine clinical practice, but it is also the foundation for clinical therapeutic trials. Two dedicated sets of response criteria are currently used both in clinical trials and—usually in simplified form—in clinical practice: the Lugano response criteria of the ICML ( ), and the RECIL criteria—a hybrid of RECIST 1.1 and the Lugano criteria—issued by the International Working Group (IWG) ( ). Both Lugano and RECIL rely on the so-called 5-point Deauville score for treatment response on FDG-PET ( ). The Deauville score is a semiquantitative approach that compares lesion FDG uptake to that of reference issues, most importantly, FDG uptake of the liver, and in the blood pool (commonly measured at the aortic arch). Following treatment, lesional uptake visually higher than in the liver (Deauville 4–5; score 5 means uptake >2 times higher than the liver background) is generally considered viable residual disease, unless there is another, noncancer-related explanation, such as infection or inflammation, postsurgical uptake, or thymic rebound. Recently, after the COVID-19 pandemic, vaccination-related FDG-avid axillary lymphadenopathy has emerged as an important cause of false-positive results ( ). While the Deauville score is officially based on the visual perception by an expert reader, radiologists in clinical practice frequently measure SUVs, especially in borderline cases; in this setting, the SUV mean of the liver represents the relevant metric for comparison.
While for FDG-avid lymphoma subtypes, the Lugano classification relies chiefly on the Deauville score, and the presence and size of a residual mass is irrelevant even for complete remission if the Deauville score is lower than 4, RECIL requires a size decrease in tumor burden of at least 30% in addition to normalization of FDG uptake (Deauville 1–3) for complete remission ( ). In general, RECIL puts more emphasis on morphological changes even in markedly FDG avid lymphoma subtypes. While the Lugano system uses subjective visible changes in FDG uptake to distinguish between partial response (PR), stable disease, and progressive disease (PD)—without guidance on changes in SUVs—RECIL applies RECIST 1.1-style morphological response and progression criteria for the same task ( ). It is also striking that, in case of a tumor burden increase of more than 20%, the lesional Deauville score does not change the diagnosis of PD, which might be crucial if checkpoint inhibitors, that sometimes lead to an immune-related phenomenon called “pseudoprogression” (i.e., size increase due to a treatment-induced influx of immune cells), are used for treatment ( ).
A notable difference between the two response classifications, which is particularly important in lymphomas with variable FDG avidity, for which FDG-PET is not recommended, also lies in the assessment of tumor burden itself. In line with WHO criteria, Lugano recommends the use of bidimensional measurements of up to six target lesions on axial CT images ( ), whereas, similar to RECIST 1.1, RECIL uses unidimensional measurements (maximum diameters) of just up to three target lesions ( ). This approach makes RECIL more practical and applicable in clinical use. On the other hand, it also means that no functional or metabolic parameter is available, which may lead to lower rates of complete remissions according to imaging, given that nonviable posttreatment sequelae may be mistaken for residual disease in such indolent lymphomas. Previous research has shown that the performance of the Lugano and RECIL systems is similar in the majority of cases ( ). It is currently unclear whether the new, provisional category of RECIL—termed minor response—is of additional clinical value.
PET/MRI in lymphoma: current status
Following years of development, PET/MRI was recently introduced as an alternative to PET/CT in oncologic imaging ( ; ; ). For lymphoma, acquisition protocols for the MRI component have generally included only a limited number of pulse sequences, in order to keep the total examination time to the minimum necessary which (also for economic considerations) would allow it to be at least moderately competitive with PET/CT in terms of patient throughput. In the authors’ institutions, for instance, the protocol consists of an axial T1-weighted Dixon, a fast coronal T2-weighted, and an axial DWI sequence, the latter obtained in free-breathing with b values of 50 and 800–900. With such a short MRI protocol, a whole-body PET/MRI with a total examination time of 30–40 min is feasible. There is currently no convincing evidence to suggest that the addition of contrast-enhanced MRI sequences or the replacement of DWI adds considerably to the diagnostic performance of FDG-PET/MRI. Therefore—and also in view of the extracosts for contrast media and the small but recognized risk of contrast reaction and possible harm in patients with impaired renal function—a set of unenhanced MRI sequences including DWI seems preferable.
In lymphoma, FDG-PET/MRI has yielded results that are comparable to those achieved with PET/CT in the majority of studies ( ; ; ; ). FDG-PET/MRI may outperform PET/CT whenever lymphomas with variable FDG avidity—in particular, MALT lymphomas—are included in the study population, and DWI is included in the MRI acquisition protocol ( ). The latter is similar to previous comparisons between whole-body DWI and FDG-PET/CT, in which PET/CT was overall slightly superior to whole-body MRI with DWI, but inferior in certain indolent subtypes for which FDG-PET is not officially recommended, as discussed above. One reason for these results is that MALT lymphoma, which is often found in tissues with considerable FDG background activity, such as the GI tract, the salivary glands, the liver, and the kidney/urinary tract, can show quite low tumor-to-background contrast, even if it is indeed FDG-avid—this is particularly true for gastric MALT lymphomas, which are detected by FDG-PET in only 9% of cases ( ). Here, PET/MRI may have an advantage over PET/CT due to the fact that, with the longer examination time of PET/MRI, selected regions of interest can be evaluated using a second, delayed time point PET, which has been shown to improve lesion conspicuity due to prolonged lesional tracer uptake on the one hand and wash out from surrounding tissues on the other hand ( ). The strong performance of DWI may also provide a second, or surrogate marker in cases where FDG-PET is needed, for example, in MALT lymphoma or CLL/SLL, to rule out transformation to DLBCL, and SUV max cut-off of five is currently recommended for this task ( ). In such cases, if FDG-PET turns out to be negative and the lymphoma is not FDG-avid, ADCs could be utilized for (semi)quantitative response assessment. In a previous study, the author’s group was able to show that DWI captures treatment response via ADC maps as early as 48–72 h posttreatment initiation in different lymphomas, in good accordance with findings on FDG-PET ( ).
To date, PET/MRI scanners—though frequently used for clinical examinations—are still quite rare outside of academic research institutions. Notably, current international lymphoma guidelines do not endorse, or even include, PET/MRI, which is possibly due to the relative novelty of the technique. Therefore, clinical trials also generally recommend PET/CT, rather than PET/MRI, though, in the author’s experience, many companies are willing to accept PET/MRI as long as the MRI protocol contains high-resolution morphological sequences that enable accurate measurement of lesion size. As has been shown in a previous study, FDG-PET/MRI leads to a dose reduction of approximately 83% compared to full dose diagnostic FDG-PET/CT, and 36% dose reduction compared to low dose PET/CT ( ; ), which is the standard especially in the United States. Therefore, PET/MRI may eventually become the hybrid imaging test of choice in radiation-sensitive populations, such as pediatric patients, who may require life-long follow-up.
Myeloma
Histology and clinical background
Monoclonal plasma cell disorders are characterized by clonal proliferation of plasma cells and accumulation of associated proteins—the latter feature being responsible for the characteristic monoclonal spike (M spike or paraprotein) on serum protein electrophoresis. According to current guidelines, symptomatic multiple myeloma—the final disease stage that includes irreversible bone destruction and has a poor prognosis—has two asymptomatic precursor stages [38]. These are (1) non-IgM monoclonal gammopathy of undetermined significance (MGUS), characterized by <30 g/L serum monoclonal protein and <10% clonal bone marrow plasma cells; and subsequently, (2) smoldering myeloma (SMM) characterized by ≥30 g/L serum monoclonal protein or ≥500 mg urinary monoclonal protein in 24 h urine, and/or 10%–60% clonal bone marrow plasma cells. Note that IgM MGUS is not considered a precursor stage of multiple myeloma, as it progresses to amyloidosis. The progression from these asymptomatic stages (MGUS and SMM) to multiple myeloma is based on the presence of so-called SLiM-CRAB criteria that list specific criteria or events that confirm the diagnosis [38]. CRAB includes
C—Hypercalcaemia
R—Renal insufficiency
A—Anemia
B—Bone lesions (lytic) on radiographs, CT, or [18F]FDG-PET/CT
The additional myeloma-defining events (MDEs)—abbreviated as SLiM—which are sometimes also referred to as biomarkers of malignancy, include
S—Sixty+ (>60)% clonal bone marrow plasma cells
Li—Light chains: involved-to-uninvolved serum free light chain ratio ≥100
M—MRI with >1 focal bone lesion of ≥5 mm size
Once any of the above MDE has occurred, treatment for multiple myeloma is started. While watchful-waiting is usually applied in non-IGM MGUS as well as SMM, multiple myeloma is treated systemically with a wide variety of anticancer drugs—including immunomodulating agents such as lenalidomide, protease inhibitors such as bortezomib, and anti-CD38 antibodies such as daratumumab—as well as stem cell transplantation and CAR-T cells ( ; ; ; ; ).
Current imaging state of the art and staging
Current guidelines by the International Myeloma Working Group (IMWG) suggest that all three major currently used imaging techniques—CT, FDG-PET/CT, and MRI—are useful for assessment of myeloma ( ; ; ; ). Apart from the availability of the respective imaging techniques, the disease stage, i.e., MGUS, SMM, or symptomatic multiple myeloma, dictates which imaging test should be used. In terms of imaging protocols, a noncontrast-enhanced, low-dose whole-body CT protocol is regarded as sufficient given the focus on osseous involvement. With regard to MRI, it is interesting to note that there is no officially recommended protocol or set of pulse sequences according to IMWG guidelines. Also, the literature has in the past been quite inconsistent, making use of noncontrast-enhanced sequences with or without DWI, and with or without contrast-enhanced sequences. This technical inconsistency may in part explain the results of comparative studies with other imaging tests such as FDG-PET/CT, which in summary do not clearly identify one technique being superior over the other. Recently, the so-called MY-RADS MRI criteria for assessment of myeloma were published, which recommend the following whole-body pulse sequences for the workup of myeloma in clinical practice ( ):
- •
Sagittal T1-weighted fast spin-echo
- •
Sagittal STIR or T2-weighted (with or without fat suppression)
- •
T1-weighted gradient-echo Dixon with fat and water reconstructions (which can be used to generate fat fraction maps)
- •
Axial DWI with two b-values (50–100 and 8–900); with STIR fat suppression.
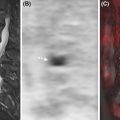
Notably, since MRI is the only technique that can directly visualize bone marrow cellular components, different patterns of marrow involvement on MRI have been identified: focal lesions; diffuse involvement; focal-on-diffuse involvement; and micronodular involvement (sometimes called salt-and-pepper pattern) ( Fig. 9.4 ).