165CHAPTER 11
Head and Neck SBRT
The use of stereotactic body radiation therapy (SBRT) in the head and neck was born out of the difficult necessity of having to manage progressive locoregional disease, from either recurrent cancer or a second primary lesion, in an area that was irradiated earlier. Oncologists have had the painful experience of treating recurrent, previously-irradiated head and neck cancer with more conventional palliative doses, only to be faced with progressive disease several months later in patients who often have nonmetastatic disease and cases in whom local therapy is quite vital. This clinical scenario poses the proverbial “rock and a hard place” in which reirradiation in the head and neck, an anatomic region characterized by numerous critical structures in close proximity including several nonredundant serial organs at risk (OARs), carries significant risks of morbidity and mortality, whereas tumor progression is notoriously insidious and universally morbid. With the rise and success of intracranial and extracranial SBRT in the late 1990s and early 2000s, SBRT became an attractive potential solution to this dilemma by allowing high-dose radiation with a reasonable likelihood of disease response and more durable control while minimizing the dose to OARs by capitalizing on the small margins and sharp dose falloff associated with SBRT.
Much of the initial research in head and neck SBRT was performed by the University of Pittsburgh group. In a series of studies, investigators systematically proved safety in the phase 1 setting and efficacy in the phase 2 setting, demonstrating the benefit of dose escalation to overcome a dose–response threshold and the ability to safely combine SBRT with systemic therapy (1–2). As a result, head and neck SBRT is becoming increasingly used in the reirradiation setting, and is more recently being explored in the up-front radiation-naïve setting for select patients who are ineligible for traditional prolonged courses of conventionally fractionated radiation therapy (RT), with or without systemic therapy (3).
SBRT is not the only reirradiation technique for recurrent or second primary head and neck cancers, as the Radiation Therapy Oncology Group (RTOG) conducted several prospective trials investigating conventionally fractionated or hyperfractionated radiation with concurrent chemotherapy (4–5). As such, optimizing patient selection for various modalities of reirradiation in general, and for SBRT in particular, is a critical need, and one in which there is a paucity of comparative research. Similarly, defining patients most appropriate for SBRT in the radiation-naïve setting remains in its infancy. Increasing publications on single-institution SBRT experiences, as well as some novel comparative research recently presented, have helped clarify which patients are most and least appropriate for head and neck SBRT and serve as a guide for “inclusion” and “exclusion” criteria when considering this treatment for a particular patient (6).
166SELECTION CRITERIA FOR SBRT: MORE APPROPRIATE PATIENTS
The original work for head and neck SBRT was performed primarily in patients who had received significant prior radiation dose to the head and neck (>30–40 Gy) and then developed either a recurrence or a second primary lesion that was unresectable in the absence of metastatic disease and required definitive management. These patients frequently present with neck disease encasing a major vessel including the carotid artery, and/or unresectable skull base disease, and are perhaps the most appropriate candidates for whom SBRT should be considered because of well representation in the evidence base. Similarly, more recent data have emerged describing the use of SBRT reirradiation in the postoperative setting—typically for gross residual disease, a positive margin, or extensive extranodal extension (7). Importantly, patients with either squamous or nonsquamous histology were included in many of these studies, as were tumors of any size or volume.
Patients in whom the use of SBRT is more controversial include patients who have resectable recurrent or second primary disease and are medically fit for surgery but refuse an operation for fear of surgical morbidity or other personal preferences. Data from the recent RTOG pattern of failure study suggest that surgical salvage was a strong predictor of improved outcomes in these patients, and SBRT is an inadequate replacement for surgery (8). That said, SBRT can be offered in this setting, albeit with clear informed consent and an acknowledgment of likely inferior outcomes. Another less studied area includes radiation-naïve patients with an initial diagnosis of head and neck cancer who are poor candidates for definitive surgical management or even conventionally fractionated radiation for 6 to 7 weeks because of advanced age, comorbidities, and/or poor social support. SBRT is an attractive option for these patients, as the shortened five-fraction treatment schedule is quite appealing and the acute toxicity quite manageable (3). Similarly, patients who present with metastatic head and neck cancer and are planned for primary systemic therapy, but have large, symptomatic primary tumors requiring up-front radiation, may be appropriate for consideration of SBRT as opposed to traditional lower dose palliative regimens (9). This may be particularly true in patients with more limited metastatic disease burden, or human papillomavirus (HPV)-positive disease, whose natural history of disease may portend extended survival (8).
SELECTION CRITERIA FOR SBRT: LESS APPROPRIATE PATIENTS
Understanding which patients are poor candidates for SBRT is a crucial need and one that continues to change as more evidence becomes available. However, several recurrent themes have emerged in the literature and can guide the judicious use of SBRT (see Table 11.1). Patients with recurrent disease, and especially those with second primaries, who are eligible and fit for resection should be recommended to proceed with surgery—SBRT is not an equivalently effective strategy. Similarly, patients with poor prognoses, who rapidly recur within 3 to 4 months of prior conventional radiation, and/or who have a life expectancy of less than 6 months, are unlikely to benefit from SBRT and should be considered for purely palliative regimens (e.g., Quad Shot; 10).
The most dramatic evidence published that demonstrated excessive harm with SBRT was a European study that demonstrated a high rate of carotid blowout syndrome (CBS) in patients with tumor encompassing more than 180° of the carotid artery (11). Although some consider this study to be a contraindication to head and neck SBRT, it resulted in modification of the dosing scheme from daily SBRT treatments to every-other-day SBRT, with the investigators demonstrating a significant decrease in adverse events (12). Many radiation oncologists continue to use SBRT in the setting of carotid encasement; however, extreme caution should be used in patients who have cancer involving all of the soft tissue between the carotid and the pharyngeal mucosa and in whom prior surgery may have created fistulas (13).
Similarly, data have emerged that suggest more SBRT-related toxicity when treating the laryngeal and hypopharyngeal regions in comparison with the neck or skull base (14). In treating these patients, one should consider tracheostomy or the placement of a feeding tube in patients with recurrences involving these areas. Patients with frank skin invasion with tumor are almost guaranteed to experience worsened soft-tissue necrosis and nonhealing wounds after SBRT and should be avoided. Although there are no established size criteria for head and neck SBRT, patients with extensive neck disease (i.e., either bilateral or multilevel ipsilateral disease) are not well suited for SBRT. Finally, although extremely rare with modern treatment planning, patients in whom disease is in close proximity to the spinal cord and/or brainstem necessitating violation of SBRT dosing guidelines should be treated with other non-SBRT–based schedules, or dose-reduced SBRT (13).
167TABLE 11.1 Factors Influencing Patient Selection for Head and Neck SBRT
More Appropriate for SBRT | Less Appropriate for SBRT |
Previously radiated patients • Unresectable recurrent/second primary disease • Tumors of skull base • Tumors of neck with minor carotid involvement/encasement • Tumors recurrent in prior flap reconstruction • Patients with >6-month life expectancy • Postoperative to high-risk area (e.g., + margin or gross extranodal extension) | Previously radiated patients • Resectable recurrent/second primary disease • Gross skin invasion • Carotid encasement with tumor communicating with mucosal surface • Diffuse neck disease (e.g., bilateral or large-volume multilevel ipsilateral) • Tumors adjacent to spinal cord and/or brainstem (may require dose reduction) • Tumors of larynx/hypopharynx region in absence of prior laryngectomy |
Radiation-naïve patients • Patients with nonmetastatic head and neck cancer who are ineligible for definitive surgery, chemo-RT, or long-course conventional RT • Patients with oligometastatic disease at presentation with symptomatic locoregional disease | Radiation-naïve patients • Patients with nonmetastatic head and neck cancer who are eligible for definitive surgery, chemo-RT, or long-course conventional RT • Patients with widespread metastatic disease |
SBRT, stereotactic body radiation therapy.
IMAGING AND TARGET DEFINITION
The extreme hypofractionation, and dose per fraction greater than 6 Gy, inherent to SBRT requires steep dose gradients that hinge on accurate target and critical OAR definition. To this end, CT simulation for SBRT planning requires fine-cut (e.g., 1.25 mm slice thickness) CT and the use of intravenous contrast enhancement to increase accuracy of target and critical OAR delineation. Complementary imaging modalities to assist in target definition have also been used in many reported experiences. For instance, 18-fluorodeoxyglucose positron emission tomography (18FDG-PET) is routinely used either at the time of SBRT simulation or via deformable image registration to CT simulation to aid in volume definition at the University of Pittsburgh, as well as in other large academic institutional experiences in the United States (15–17). Although others have used gadolinium-enhanced fine-cut magnetic resonance imaging (MRI) fused to the CT simulation to similarly increase the accuracy of target definition (18–20), clinically, we find MRI most useful when treating recurrent volumes close to the base of skull or paranasal sinuses, in which proximity to high-risk neural structures (e.g., temporal lobes, brainstem, optic nerve, and optic chiasm) necessitates this modality to aid in critical OAR and target definition (Figure 11.1). To account for organ motion, such as when treating the larynx, four-dimensional CT scans may be used. However, few reports have described such methodology acknowledging that although the motion of the larynx can be significant, the time during which this motion takes place is small relative to total treatment time (21, 22). Others have used intrafraction monitoring techniques such as ExacTrac® (BrainLab, Munich, Bavaria) or CyberKnife® (Accuray, Sunnyvale, California) six-dimensional skull or spine tracking to account for such potential motion during treatment delivery (13).
When performing target definition, we recommend the following steps:
1. There should be collaboration between a radiation oncologist, neuroradiologist, and head and neck surgeon for multidisciplinary target definition while taking into account the patient’s medical history, anatomy (which is commonly distorted in the setting of recurrent disease), and potential patterns of spread.
2. Information should be incorporated from both contrast-enhanced CT simulation and complementary imaging modalities such as PET/CT or gadolinium-enhanced fine-cut MRI (Figure 11.2A–C).
3. We do not routinely incorporate a clinical target volume (CTV) margin for treatment of the primary lesion, nor do we routinely electively irradiate nodal levels when using SBRT for recurrent head and neck cancer. This practice is consistent with recommendations for conventional reirradiation and is imperative to minimize risks of complication with high-dose-per-fraction reirradiation with SBRT (23).
4. Although many of the early reports using SBRT in head and neck cancer extrapolated from clinical experience with intracranial stereotactic radiosurgery (SRS) and focused on the gross tumor volume (GTV) with no additional margin for the planning target volume (PTV), many radiation oncologists now use a GTV to PTV expansion despite use of these advanced imaging modalities consistent with patterns of failure analyses with PET/CT-based SBRT experiences (13, 24). However, although PTV is not modified by strict definition, a reduced or edited PTV may be permitted in cases in which the PTV margin significantly overlaps with critical OARs to respect normal tissue tolerances in the reirradiation setting (Figure 11.2D–F).
168
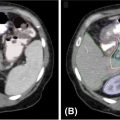
FIGURE 11.1 Example of the importance of additional high-resolution imaging such as MRI or PET/CT fusion or simulation in target definition for SBRT. (A) CT with true extent of GTV especially with extension toward brainstem and right temporal lobe difficult to assess; (B) 1.25-mm slice thickness, high-resolution, contrast-enhanced, T1-weighted MRI with GTV highlighted in red. The red GTV volume is then displayed over the CT-fused image to highlight the importance of this imaging and fusion in SBRT treatment planning.
GTV, gross tumor volume; SBRT, stereotactic body radiation therapy.
DOSE FRACTIONATION SCHEMES
Various dose fractionation schemes have been used and described in the literature. Most treatment regimens are largely empirical with clinician experience and preference dictating the regimen used. SBRT for head and neck cancers is largely used in the reirradiation setting. This is unlike other sites, such as SRS for intracranial lesions and SBRT for early stage lung cancers, in which the treated lesions are in previously unirradiated tissue. Dose–response relationships and toxicity data are well studied and more mature for these situations.
The unique challenge posed by the head and neck population is the fact that data for normal tissue toxicity with high-dose-per-fractionation RT in earlier irradiated tissues are scarce. Most of the guidelines and recommendations present unvalidated data for a few structures (13). In these recommendations, previous dose of radiation to tissues and structures and the time since previous radiation are not considered. Radiation dose tolerance information for a large number of OARs such as the brain, bone, carotid artery, cranial nerves, skin, and soft tissue is unknown. In addition, dose–response relationships for recurrent tumors in the earlier operated or irradiated tissue with underlying hypoxia and fibrosis are also largely unknown. Thus, dose fractionation schemes for SBRT in recurrent head and neck cancers are still evolving.
Review of the literature reveals a wide array of schemes (see Table 11.2). Some centers had earlier used one large dose of 10 to 18 Gy in one fraction similar to the paradigm used for SRS for intracranial lesions. However, most centers started fractionating treatments to minimize side effects and to increase the delivered dose over a period of time achieving up to eight fractions for dose delivery. The Centers for Medicaid and Medicare Services (CMS) allows for a maximum of five fractions to be classified as a SBRT treatment. With this standardization, most centers in the United States now use a maximum of four to five fractions to deliver doses ranging from 20 to 44 Gy.
169
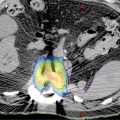
FIGURE 11.2 Volume definition using PET/CT-based simulation: (A) GTV delineation on the basis of CT in green; (B) FDG PET/CT GTV in red; (C) combination of PET and CT information used to define GTV contour; (D) 2-mm PTV expansion in orange (notice editing of PTV at interface with mandible in yellow); (E) 40 Gy in five-fraction color wash; and (F) 20 Gy in five-fraction color wash (notice skin and ipsilateral parotid sparing).
FDG, fluorodeoxyglucose; GTV, gross tumor volume.


170One systematic approach to this problem was reported by Heron et al. in 2009 (1). The investigators at the University of Pittsburgh conducted a phase I trial in 25 patients with recurrent head and neck cancers. Escalating doses ranging from 20 Gy in five fractions to 44 Gy in five fractions were studied. Even at the highest dose level, no grade 3 to 4 or other dose-limiting toxicities were noted. A subsequent publication by the same group treated patients with doses up to 50 Gy in five fractions (15). An improvement in locoregional control was noted in patients receiving between 40 and 50 Gy as opposed to those who had received 15 to 36 Gy. In addition, tumors measuring greater than 25 mL in volume had inferior locoregional control as opposed to those that were 25 mL or less. Locoregional control improved in both groups with increasing delivered dose.
171
FIGURE 11.3 Local control probability against prescribed dose. Pooled data from the literature reporting local control and dose at 1, 2, and 3 years, as shown. Each data point represents one dataset, and doses are computed (via the linear–quadratic mode) as “five-fractional equivalent total doses” (patient numbers of each study are in parentheses in the keys of each figure panel). Vertical error bars are 68% binomial confidence intervals. Solid line is the logistic model; dashed lines are 95% confidence intervals for the dose–response.
Source: Vargo JA, Moiseenko V, Grimm J, et al. Head and neck tumor control probability: radiation dose-volume effects in stereotactic body radiation therapy for locally recurrent previously-irradiated head and neck cancer: report of the AAPM working group. Int J Radiat Oncol Biol Phys. 2018. doi: 10.1016/j.ijrobp.2018.01.044
In an attempt to further improve the local control and survival outcomes in this group of patients, systemic therapy in combination with SBRT has also been studied. The University of Pittsburgh group initially reported on a retrospective matched cohort study of 70 patients, 35 (50%) of whom had received cetuximab infusion during SBRT treatment (26). The dose of radiation was 40 to 44 Gy delivered over five fractions on alternating days. Cetuximab was started at a dose of 400 mg/m2 1 week prior to the start of SBRT and continued at the dose of 250 mg/m2 during SBRT. The combined complete response (CR) or partial response (PR) rate for the group of patients receiving cetuximab was 77.1%, whereas it was 62.9% in the SBRT-alone group. The median overall survival was also noted to be higher with the addition of cetuximab to SBRT (24.5 months vs. 14.8 months for SBRT-only patients, respectively). This was subsequently followed by a prospective phase 2 trial with the same regimen for SBRT and cetuximab (2). The rate of 1-year local progression-free survival was 60%, with a median overall survival of 10 months among the 50 patients enrolled in the study. A similar multi-institutional prospective phase 2 study was also conducted in Europe (18). Cetuximab was administered in the same manner as mentioned earlier; however, the dose of radiation was 36 Gy in six fractions over 11 or 12 days. The CR plus PR rate was 69.4%, with a median overall survival of 11.8 months and a 1-year survival rate of 47.5%.
SBRT FOR NEWLY DIAGNOSED HEAD AND NECK CANCERS
Although SBRT has been used primarily in the treatment of recurrent or second primary head and neck cancers, there are encouraging data on its use for the treatment of newly diagnosed cancers when other standard therapy is unsuitable. A report from Henry Ford Hospital described 11 patients treated with SBRT for various histologies (27). Doses ranged from 18 Gy in one fraction to 48 Gy in 16 fractions. A combined CR plus PR rate of 82% was noted with 1-year local control and overall survival rates of 83% and 70%, respectively. Similar high combined response rates of 84% were seen in another report on 13 radiation-naïve patients who received 35 Gy in five fractions (28). Among those who had been earlier irradiated to the same area, the response rates were lower at 62%. In a more recent publication, 12 elderly patients with a median age of 88 years were treated to a dose of 44 Gy in five fractions (3). Three of these received cetuximab in addition to SBRT. The 1-year local control was 69%.
DOSE TO CRITICAL STRUCTURES AND TOXICITY OF TREATMENTS
The overall toxicity profile of head and neck SBRT has been favorable when compared with conventionally fractionated reirradiation with or without concurrent systemic therapy. The rates of acute grade 3 to 4 toxicity and treatment-related mortality for conventional RT, even with the use of modern intensity-modulated radiation therapy (IMRT) techniques, are approximately 20% and 1725% to 7%, respectively (29). In comparison, the rates for the same end points are approximately 10% and less than 1%, respectively, for SBRT. The addition of cetuximab did not appear to increase toxicity in the aforementioned studies.
Some of the unique toxicities associated with SBRT are as follows.
Carotid Blowout Syndrome
CBS is a potentially fatal complication in patients with head and neck cancers. It refers to the rupture and hemorrhage from the carotid artery or its major branches as a result of tumor invasion or because of aggressive treatment-related complications. CBS is an often confusing entity. It is carotid rupture in the absence of uncontrolled disease, suggesting a complication of treatment. Unfortunately, the literature is confounded by a mixture of uncontrolled disease resulting in ruptures. The reported mortality rates range from 20% to 100%, with a similarly high rate of major neurological complications (30). A 2.6% risk of carotid artery blowout after conventional reirradiation is reported, approximately 76% of which are fatal (31).
Unfortunately, the risk of CBS is much higher in patients treated with high-dose-per-fraction RT. In a series reported by Cengiz et al., 46 patients underwent reirradiation to a dose of 30 Gy (range, 18–35 Gy) in a median of five fractions (range, one to five fractions; 11). Eight (17.3%) of these had a carotid blowout with death occurring in seven. On the basis of this analysis, the authors reported that CBS occurred in patients whose tumor encased the carotid artery by 180° or more, or cases in which the carotid artery received 100% of the prescribed dose. The majority of CBS occurred within the first 6 months after treatment. In a study from Japan reporting on 381 patients reirradiated to the same median dose of 30 Gy in five fractions after previous median RT dose of 60 Gy, the CBS rate was 8.4% (32). This proved fatal in 22 patients. On univariate analysis, older age, skin invasion, and necrosis or signs of infection were predictive for CBS. However, only skin invasion was significant on multivariate analysis. In a subsequent matched-pair analysis by the same group, the following three factors were identified as being predictive of CBS: carotid involvement of greater than 180° by tumor, presence of ulceration in the tumor, and lymph nodal irradiation. The presence of no, one, or two risk factors resulted in a CBS-free survival of 100%, 95%, and 84%, respectively, at 12 months. However, the CBS-free survival at 6 months was only 25% in the presence of all three factors (33). A more recent report further emphasized the important negative prognostic implication of tumor-related mucosal ulceration in causing CBS at a median of 4.2 months after reirradiation (34).
In addition to the tumor-related factors, the treatment delivery regimen also appears to significantly impact the incidence of CBS. Yazici et al. reported on their institutional experience among 75 patients in which the first group of 43 patients received high-dose-per-fraction RT on a daily basis (12). CBS was observed in 16% of these patients with a CBS-free median survival of 9 months. A change in treatment delivery to an alternate-day regimen in the subsequent 32 patients reduced the rate of CBS to 12.5%, with a significant increase in CBS-free survival to 23 months.
In summary, the following factors seem to increase the risk of fatal carotid artery blowout: (a) tumor involving the artery greater than 180°; (b) mucosal ulceration because of tumor; (c) skin involvement; (d) nodal irradiation; and (e) treatment delivery on consecutive days. Careful patient selection with multidisciplinary care of this complication, should it occur, is essential to avoid treatment-related mortality. It should be noted that CBS is not used as a contraindication to SBRT by all institutions, as large, single-institutional, dose–volume analyses have not demonstrated excess toxicity in patients treated with this configuration with no correlation between CBS and carotid dose (35). Additionally normal tissue complication models have been generated to guide the application of SBRT against carotid artery doses (51) (Figure 11.4).
Spinal Cord Myelopathy
Spinal cord myelopathy is rarely encountered in published reports on head and neck SBRT. This is in large part because of two major factors. First is the careful planning and maximum dose restriction to less than 45 to 50 Gy to the spinal cord during the initial course of SBRT. Second, there are robust experimental and clinical data providing information on spinal cord dose tolerance to reirradiation.
SBRT to the spinal and paraspinal region results in sharp dose gradients with partial volume exposure of the spinal cross-section to any high dose of radiation. Experimental data from spinal cord radiation in pigs demonstrated a 50% incidence of motor nerve deficits for a maximum point dose of 19.7 Gy after a previous dose of 30 Gy in 10 fractions delivered 1 year prior (36). No neurological compromise was reported at doses less than 18.8 Gy, whereas doses greater than 21.3 Gy resulted in 100% toxicity.
173
FIGURE 11.4 Probability of carotid toxicity. Pooled data predictive of 1-, 2-, and 3-year probability of local control on the basis of dose. Note that the increasing impact of higher dose on local control is heightened with longer follow-up, that is, 3 years > 2 years >1 year.
In humans, doses of 5 to 6 Gy × 5 fractions or 8 Gy × 3 fractions appear to be safe after previous 30 Gy in 10 fractions when the spinal cord surface point dose is restricted to the maximum prescribed dose (24–30 Gy; 37). A meta-analysis also showed very low rates of spinal myelopathy with reirradiation among many centers that had published earlier on their spine SBRT experience (38). Nieder et al. developed risk stratification on the basis of cumulative biologically equivalent dose (BED), duration between the two courses of radiation, and the BED of one of the two courses of RT being 102 Gy or greater in 2 Gy per fraction. On the basis of the sum of these risk scores, patients are divided into low-, intermediate-, and high-risk groups with the risk of myelopathy at 3%, 25%, and 90%, respectively (39).
Although the validity and use of the linear–quadratic (LQ) model and BED calculations at high dose per fraction is a matter of intense debate and speculation, Sahgal et al. used this methodology to determine spinal cord dose tolerance to reirradiation. The investigators performed a multi-institutional dose–volume histogram (DVH) comparison between 14 patients who did not develop radiation myelopathy after reirradiation with SBRT and five patients who did (40). They have developed recommendations for the maximum point dose to the thecal sac allowed for a planned course of SBRT after previous external beam RT to the spinal cord. On the basis of their data, a minimum of 5 months must be allowed between the two courses. For example, the maximum acceptable dose to the thecal sac is 15.5 Gy in five fractions after a previous dose of 50 Gy in 2 Gy per fraction to the same segment of the spinal cord.
Brain Necrosis
Similar to the reasons stated earlier for spinal cord toxicity, brain necrosis is also rarely reported in patients undergoing SBRT. The Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) guidelines provide an excellent review for the dose–volume constraints in the brain for up-front and reirradiation cases (41). No cases of brain necrosis were observed when the total cumulative BED was less than 100 Gy in 2 Gy per fraction (42).
Other Toxicities
A variety of other toxicities have also been reported in published literature. Local rates of grades 1 and 2 toxicity are reported in approximately 100% of treated patients (27).
Grades 3 and 4 toxicities are reported to varying degrees in acute and late settings. One of the major drawbacks is the retrospective nature of these reports, which limits the validity of reported toxicities. However, the serious and significant toxicities include mucositis, dysphagia, ulceration of skin or mucosa, fistula formation, bone and soft-tissue necrosis, xerostomia, cranial nerve palsy, and hemorrhage (see Table 11.3). Unfortunately, it is very difficult to draw any meaningful dose–volume–structure data for the incidence of these toxicities because of the lack of such information in the published reports.
Carotid artery blowout or hemorrhage from other major blood vessels is a major cause of mortality. However, with increasing experience with the use of SBRT in recurrent head and neck cancers, mortality rates are very low or rarely seen in more contemporary series.
In one recent article, grade 3 or greater acute and late toxicities were retrospectively evaluated in 291 patients (14). Various disease- and treatment-related factors were considered for analysis including site of recurrence, postoperative versus nonpostoperative cases, prior dose of RT, chemotherapy, and treatment volume. Acute grade 3 or higher toxicity was noted in 11.3% of patients. Grade 3 side effects were dysphagia, mucositis, skin toxicity, trismus, xerostomia, and fatigue. Acute grade 4 pharyngeal edema and lingual artery bleed were seen in one patient each. One treatment-related mortality was seen shortly after completion of treatment because of cardiac arrest. Grade 3 or higher late toxicity was higher at 18.9% with treatment-related mortality in seven patients (three patients with carotid blowout). Treatment of lymph node recurrence was associated with a lower risk of acute toxicity. SBRT dose of 44 Gy or higher in five fractions delivered on alternate days resulted in significantly more grade 3 or higher acute and late toxicities. When examining the sites of treatment, patients receiving treatment to the laryngeal/hypopharyngeal region had significantly higher acute (20%) and late (50%) toxicities than those receiving treatment to other head and neck sites.
174
175CASE STUDY
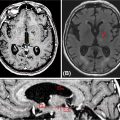
A 70-year-old male patient had previously been treated for carcinoma of the nasopharynx at an outside institution. He had been treated to a dose of 70 Gy in 35 fractions with concurrent cisplatin chemotherapy followed by adjuvant chemotherapy with cisplatin and 5-fluorouracil. He presented with symptoms of severe headaches and pain on the right side of his face 2 years after his previous treatment. He was noted to have a recurrence in his nasopharyngeal/parapharyngeal region. A review of the prior RT plan revealed that the area of the recurrence has received between 70 and 72.5 Gy. This lesion was PET avid and confirmed on T1-weighted contrast-enhanced MRI. This recurrent lesion was treated using SBRT to a dose of 30 Gy in five fractions prescribed to the 95% isodose line. A repeat MRI completed 4 months posttreatment showed significant reduction in the size of the mass with symptomatic relief of his symptoms (Figure 11.5). The patient developed metastatic disease in the lungs after 6 months and passed away with locally controlled disease.
Treatment Planning and Plan Evaluation
Although many of the initial studies have favored treatment delivery platforms that aggressively used multiple noncoplanar treatment plans such as the CyberKnife platform (Accuray), the majority of the authors now favor linear accelerator (linac)-based multibeam delivery with 10 to 15 coplanar beams or two to three arcs with volumetric-modulated arc therapy. This helps to reduce treatment 176time in a patient population, often with difficulty tolerating long treatment times because of airway compromise, swallowing dysfunction, or pain (13). However, non-coplanar beams are often useful when treating lesion in close proximity to the skull base. When evaluating plan quality, it is important to watch for streaking phenomenon (see Figure 11.8C), which can increase skin toxicity as the optimizer is pushed to create sharp dose gradients.

FIGURE 11.5 Case example of SBRT for locally recurrent nasopharyngeal cancer. Highlighted is the SBRT plan delivering 30 Gy in five fractions with pre- and post-SBRT MRI showing complete clinical response.
SBRT, stereotactic body radiation therapy.
OUTCOME DATA
Outcome data from what is primarily retrospective supportive literature are outlined in Table 11.2. More recently, a number of single-institutional prospective datasets have been published to better define the expected control and toxicity associated with SBRT for reirradiation of recurrent squamous cell carcinoma of the head and neck. The prospective phase I dose escalation study previously mentioned by Heron et al. first established the safety of dose escalation of SBRT from 25 to 44 Gy over five fractions delivered on alternating days over 1 to 2 weeks with no reported dose-limiting toxicity; however, this dataset also highlighted the suboptimal outcomes with low-dose SBRT without radiosensitizing systemic therapy with a response rate of 17% and median overall survival of 6 months (1). Building on the landmark data from the primary definitive treatment of newly diagnosed squamous cell carcinoma of the head and neck, in which integration of concurrent cetuximab improved outcomes compared with RT alone, cetuximab has been shown to have an important therapeutic benefit when added to SBRT for recurrent squamous cell carcinoma of the head and neck (43). Investigators from Lille, France, initially reported a small prospective feasibility study of SBRT (36 Gy in six fractions) with concurrent cetuximab, which showed promising results with the addition of cetuximab to SBRT with a response rate of 79%, a median overall survival of 14 months, and only a 10% rate of grade 3 toxicity (44). These results have been further validated by two prospective phase II studies. The first, a French multi-institutional study, which again used 36 Gy in six fractions plus cetuximab, confirmed the results from their previously published feasibility study showing a response rate of 58%, 1-year overall survival of 48%, and a grade 3 or higher toxicity rate of 30% (19). The second was a phase II prospective study from the University of Pittsburgh, which used 40 to 44 Gy in five fractions with cetuximab, with a similar 1-year overall survival of 40% and grade 3 or higher toxicity of 12% (2). These experiences set SBRT and cetuximab as a viable reirradiation strategy for recurrence of squamous cell carcinoma of the head and neck. Recently, a large multi-institutional comparative effectiveness project has been reported comparing reirradiation with SBRT with and without cetuximab with conventional fractionation with and without chemotherapy with modern IMRT techniques (6). Although limited by the retrospective study design, the results suggest relative equipoise between the two modern reirradiation techniques in terms of tumor control, especially for patients reirradiated within 2 years with preexisting organ dysfunction (e.g., tracheostomy or feeding tube), further supporting the potential role of SBRT in the treatment armamentarium for recurrent squamous cell carcinoma of the head and neck, especially recognizing the decrease in treatment time and burdens of care associated with SBRT (45). Ongoing trials are further exploring the role of SBRT in recurrent head and neck cancer integrating recently approved novel systemic agents such as anti-PD-1 immunotherapy and pembrolizumab (KEYSTROKE Trial), or the addition of adjuvant systemic therapy with docetaxel and cetuximab (NCT 02057107).
QUALITY OF LIFE OUTCOMES AND FOLLOW-UP
Uncontrolled disease in the head and neck carries a significant risk to a number of vital functions including mastication, swallowing, speech, airway protection, and risks of fatal bleeding. Although reirradiation with SBRT may not always provide long-term cure for the majority of patients, reirradiation can palliate a number of these distressing symptoms. However, the risks of toxicity with aggressive salvage strategies such as SBRT must be balanced against the risks to an often fragile patient’s quality of life. In the largest prospective evaluation of patient-reported quality of life, investigators from the University of Pittsburgh used the previously validated University of Washington Quality of Life Revised Questionnaire from 2004 to 2011 to quantify the impact of reirradiation with SBRT with and without cetuximab on patient-reported quality of life (46). Following an initial 1-month decline, SBRT with and without cetuximab significantly improved patient-reported overall quality of life as well as specific 177domains of swallowing, speech, saliva, activity, and recreation relative to baseline independent of age, use of cetuximab, tumor volume, and reirradiation interval (46). Similarly, 62% of patients reported stable or improved quality of life following reirradiation with SBRT with cetuximab in a prospective trial of SBRT with cetuximab (2). Combined, although comparative effectiveness of SBRT with other salvage strategies such as systemic therapy or conventional fractionated reirradiation is currently lacking, these data speak to the palliative benefits of SBRT wherein short treatment time and low rates of acute toxicity make SBRT an attractive reirradiation strategy for recurrent head and neck cancers. Follow-up after SBRT remains consistent with clinical practice following conventional RT for head and neck cancer with baseline PET/CT or MRI 3 to 4 months after the completion of therapy and close clinical follow-up with a comprehensive head and neck exam every 3 months (see Figure 11.5). There is heterogeneous practice in regards to additional imaging follow-up after the 3- to 4-month PET/CT, as these patients remain at high risk for recurrence (13).
SAFETY CONSIDERATIONS
Inherent to the extreme hypofractionation of SBRT and concerns of reirradiation toxicity, many concerns of risks of late toxicity following SBRT in recurrent head and neck cancer have been raised. In the only available comparative effectiveness analysis of reirradiation for recurrent head and neck cancer comparing SBRT with IMRT, SBRT appeared to result in potentially lower acute toxicity and comparable late toxicity than IMRT (6). When implementing SBRT, however, some unique safety considerations apply:
1. CBS: Fatal bleeding events are often the most feared complication of reirradiation of head and neck cancers. As described earlier, a number of clinical factors such as tumor involving the artery greater than 180°, mucosal ulceration because of tumor, skin involvement, nodal irradiation, and treatment delivery on consecutive days have been suggested to predict for higher risk of CBS (33–36). It is important to note that uncontrolled tumor in the head and neck also places patients with recurrent head and neck cancer at high risks of fatal bleeding event; thus, often despite extensive carotid involvement, a decision between the patient and the treating team may be made to accept the risks of retreatment. When treating such patients, it is important to watch for development of necrosis or infection of the treatment site and attempt to spare the carotid as much as possible without compromising tumor coverage (Figure 11.6).