Head Trauma
4.1 Introduction and Epidemiology
The incidence of head trauma requiring hospitalization is estimated at 200 to 300 cases per 100,000 population per year. Children under 15 years old account for approximately one-fourth of all cases. Accident-related head injuries are among the leading causes of death in children and young adults. Head trauma is the dominant factor in approximately one-half of all fatal accidents.
The nature and extent of permanent neurologic impairment depend on how much brain tissue has been damaged and what brain areas have been affected. But not all head injuries causing permanent neurologic deficits leave morphologic traces that are detectable by imaging studies.
4.2 Classification and Clinical Grading
Grading the severity of head trauma is based not on radiologic findings but on clinical criteria. Head injuries are traditionally classified into three grades—mild, moderate, or severe—based on the duration of initial unconsciousness or the time needed for neurologic recovery. A more recent approach is to grade head injuries based on initial neurologic findings using the Glasgow Coma Scale. Patients are scored on the basis of eye opening, verbal response, and motor response. The total score ranges from 3 points (no responses) to 15 points (normal responses). A score of 3 to 8 indicates severe head injury, 9 to 12 moderate injury, and 13 to 15 mild injury. If there was no initial loss of consciousness, the injury is classified as minimal (subconcussive) head trauma. The classification of a head injury as open or closed depends on the integrity of the dura mater.
The intracranial effects of head trauma are subdivided into primary and secondary lesions:
Primary lesions: These lesions occur at the time of injury, even if considerable time passes before they are fully manifested. They may be caused by direct contact (e.g., the contusion of brain tissue by indriven bone fragments) or indirectly by centrifugal forces (e.g., relative motion between the brain and skull or shearing forces along nerve fibers). Whether treatment can prevent a primary lesion from causing neurologic dysfunction depends on the type of lesion. Shearing injuries or contusions, for example, respond poorly to treatment whereas an epidural or subdural hematoma is often treatable by surgical decompression.
Secondary lesions: These secondary effects of head injuries develop as complications of the primary trauma. In principle, secondary lesions can be prevented if the underlying injuries are promptly detected and appropriately treated.
It is also helpful to subdivide head injuries into intra-axial lesions (occurring in the brain tissue itself) and extra-axial lesions (e.g., involving the ventricular system or meninges). Traumatic vascular injuries are another important concern. The principal traumatic lesions are reviewed in ▶ Table 4.1.
Traumatic lesions | Extra-axial lesions | Intra-axial lesions |
Primary traumatic lesions |
|
|
Primary traumatic vascular lesions |
| |
Secondary traumatic lesions |
|
|
4.3 Magnetic Resonance Imaging of Head Trauma
4.3.1 Role of MRI in Trauma Diagnosis
The prognosis of head trauma depends critically upon how swiftly neurosurgical treatment can be instituted. Thus, the initial workup of head trauma should quickly establish the extent of intracranial injuries and, first and foremost, should reliably detect lesions that require immediate causal therapy. The most important of these are epidural and subdural hematomas and depressed skull fractures. CT is an excellent modality for the detection of these lesions.
Note
CT is the modality of choice for the initial evaluation of head trauma and should be performed without delay.
Although the primary effects of head trauma, excluding skull fractures, can also be detected by MRI and the scan time can be shortened to less than 15 minutes by using ultrafast sequences, MRI is not used during the acute phase after a head injury because of its limited availability and the limited options for patient surveillance. The only exception to this rule is in patients with a suspected concomitant intraspinal lesion or vascular dissection. Although comparative studies found that MRI was slightly more sensitive than CT in the detection of extra-axial hematomas, the hematomas missed by CT were generally very small and did not require surgical intervention. Even in patients showing acute deterioration of neurologic status during further treatment, CT is generally preferred over MRI.
Note
Head-injured patients should always undergo MRI if their neurologic status is not explained by CT findings.
MRI is much more sensitive than CT in the detection of shearing injuries, small contusions, treatable pontine myelinolysis, and other brainstem lesions. MRI is also better for evaluating the various stages of rebleeding, and it can provide forensic documentation in patients who will later be referred for disability evaluation. MRI is also superior to CT for defining the full extent of a traumatic brain injury when performed within 2 weeks after the trauma.
4.3.2 Examination Technique
Most traumatic lesions can be detected with an MRI protocol consisting of T1w, T2w, T2*w, and FLAIR sequences in at least two mutually perpendicular planes. Spin echo (SE) or turbo spin echo (TSE) sequences are most commonly used for T2w imaging. The refocusing pulse makes these sequences less sensitive to local magnetic field inhomogeneities, so that image contrast is determined almost entirely by the T2w contrast. This may actually be a disadvantage in trauma imaging, however, as the detection of small hemorrhages relies on the local magnetic field disturbances caused by susceptibility artifacts. Consequently, the use of T2*w sequences, which are very sensitive to these effects, is considered essential in trauma examinations. Moreover, edematous parenchymal lesions are often more difficult to detect on T2w SE or TSE images than on FLAIR or PDw TSE images. Diffusion-weighted imaging (DWI) can aid in the detection of shearing injuries and secondary ischemic lesions. Studies are currently under way to determine the efficacy of diffusion tensor imaging (DTI) for the investigation of shearing injuries and of perfusion imaging for raised intracranial pressure. Perfusion imaging can also demonstrate contusional brain lesions, often in an early phase not yet detectable by unenhanced cranial CT.
4.3.3 MRI Detection of Intracranial Hemorrhage
The reliable detection of intracranial hemorrhage is of prime importance in the investigation of head injuries. Contrary to initial expectations, MRI has proven to be very sensitive in the detection of hematomas. The signal characteristics of intracranial hematomas on MRI depend mainly on the age of the collection. They are determined chiefly by the oxidative state of hemoglobin (oxyhemoglobin, deoxyhemoglobin) and its breakdown products (methemaglobin, hemosiderin, ferritin). Traumatic intracerebral hematomas behave much like any other hematomas in this regard. The main signal characteristics are summarized in ▶ Table 4.2 (see also ▶ Table 2.1). These principles can also be used to determine the approximate age of a hematoma.
Stage of hematoma | Time since injury | Pathophysiology | Hemoglobin | T1w SE | T2w SE | T2*w GRE |
Hyperacute | <6 h | Extravasation of blood | Intracellular oxyhemoglobin | Hypointense to isointense | Hyperintense | Hypointense |
Acute | 6 h to 3 days | Deoxygenation | Intracellular deoxyhemoglobin | Hypointense to isointense | Hypointense | Very hypointense |
Subacute:
| 3–7 days | Clot shrinkage and conversion of deoxyhemoglobin to methemaglobin | Intracellular methemaglobin | Hyperintense, may have hypointense center | Hypointense | Very hypointense |
| 7 days to months | Lysis of erythrocytes | Extracellular methemaglobin | Hyperintense | Hyperintense, often with hypointense rim | Hypointense |
Chronic | Months to years | Clearing of the hematoma by macrophages | Hemosiderin and ferritin | Hypointense | Hypointense | Hypointense |
The above signal characteristics apply to imaging at 1.5 T, and paramagnetic effects may be much weaker at lower field strengths. The duration of the individual stages may vary considerably from case to case. | ||||||
Even in the hyperacute stage, within a few hours, intracerebral hematomas can be reliably detected by T2*w sequences, which are sensitive to susceptibility artifacts. Fresh blood already appears partially hypointense at this stage. Most hematomas are also manifested by local mass effect and perifocal edema. Another sign of intracerebral hematoma, detectable even at an early stage in T2w or T2*w sequences, is a narrow hypointense rim caused by peripheral deoxygenation.
Tips and Tricks
MRI is definitely superior to CT for detecting hemorrhages older than 2 weeks, and T1w images can often detect methemaglobin for months. Hemosiderin deposits are still detectable in T2w and SWI sequences for many years.
The principles described for intraparenchymal hematomas also apply to extra-axial hematomas, although there are some special features that depend on the affected anatomic compartment. They are discussed in the relevant sections.
4.3.4 Prognostic Value of MRI
Sectional imaging by CT or MRI has become a standard tool for treatment planning and follow-up in head-injured patients and has significantly improved their prognosis. Despite many attempts, however, CT cannot reliably predict prognosis after a head injury. This is because CT is too insensitive to shearing injuries and brainstem lesions, which greatly affect the prognosis.
MRI can provide a more accurate neurologic prognosis than CT. Primary brainstem injuries and extensive shearing injuries with involvement of the corpus callosum are reasonably good predictors of a poor prognosis. Injury to the corpus callosum in itself is not directly responsible for neurologic deficits and provides only an indirect marker for the severity of shearing injuries. The number of individual shearing lesions, regardless of their location, also correlates inversely with neurologic prognosis. On the other hand, other supratentorial lesions, extra-axial hematomas, and cerebral contusions have a definite poor prognosis only if they have led to transtentorial herniation with secondary brainstem damage. Even patients with transtentorial herniation and moderate brainstem compression may recover if the cause of the herniation can be surgically corrected without delay and the brainstem is undamaged.
Firsching et al showed in several studies that more than half of head-injured patients who did not lose consciousness had a brainstem lesion that was not detectable by CT. They found that prognosis could be predicted with relatively high confidence when brainstem damage was classified by anatomic location. For example, they found that all patients with bilateral pons lesions died, and that a high percentage of patients with bilateral lesions of the mesencephalon were in a persistent vegetative state. Patients who had a unilateral brainstem lesion on MRI or displayed only supratentorial lesions had a good chance of surviving with little or no disability. The mortality rate was 4% in patients without brainstem injury and 41% in patients with brainstem injury. The likelihood of surviving unconsciousness without disability was 70% in patients without brainstem injury and 22% in patients with unilateral brainstem injury. Bilateral brainstem injury almost invariably led to permanent neurologic impairment. Firsching et al could not distinguish between primary and secondary brainstem lesions because MRI in their study was performed up to 8 days after trauma. Given this delay, it is reasonable to assume that secondary brainstem damage due to herniation was not uncommon in their study.
Note
Brainstem lesions critically affect the prognosis in head-injured patients. It has been shown that MRI permits an accurate prognostic assessment during the subacute phase after trauma.
Studies are currently under way to determine whether new MRI techniques such as DWI and DTI can supply additional useful prognostic information.
4.4 Primary Traumatic Lesions
4.4.1 Skull Fractures
Skull fractures are present in approximately two-thirds of patients with severe head trauma. They are classified morphologically as linear fractures, comminuted fractures, depressed fractures, or perforating fractures (gunshot and stab injuries). The intracranial displacement of bone fragments detected radiographically in a depressed fracture may not equal the maximum displacement that occurred at the time of injury. Fractures are clearly depicted on plain radiographs and CT scans. There are isolated reports of skull fractures also being detectable by MRI, which can demonstrate fluid or blood in the fracture gap and can also detect bone edema when suitable techniques (e.g., STIR sequences) are used. The detection of skull fractures by MRI is not relevant in everyday clinical practice, however.
4.4.2 Epidural Hematoma
4.4.2.1 Acute Epidural Hematoma
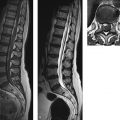
Epidemiology and pathology Epidural hematomas are found in approximately 5% of patients imaged for head trauma. By far the most frequent cause (90% of cases) is a skull fracture, typically located in the frontoparietal region. The sharp-edged fragments may lacerate meningeal arteries (60% of cases, usually branches of the middle meningeal artery) or venous vessels (40%; middle meningeal vein, a dural sinus, or diploic veins). The extravasating blood collects between the inner table and dura, often forming a biconvex mass ( ▶ Fig. 4.1). Spread of the hematoma along the calvarium is almost always limited by sutural lines, as the dura is firmly adherent to the bone at those sites and is fused with the endosteum. The great majority of epidural hematomas are unilateral (95%) and supratentorial (95%).

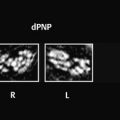
Fig. 4.1 Epidural hematoma in the posterior cranial fossa. MRI examination 10 days after head trauma demonstrates a left-sided, biconvex epidural hematoma in the posterior fossa. Having reached the late subacute stage, the hematoma appears predominantly hyperintense in both T1w and T2w sequences. The dura appears as a thin hypointense line between the hematoma and cerebellar hemisphere. (a) Axial T2w TSE sequence. (b) Axial T1w TSE sequence.
Note
A bilateral epidural hematoma that crosses the sagittal suture generally results from a fracture-related injury of the sagittal sinus. MR images should be checked in these cases to determine whether the sinus is still perfused.
MRI and CT findings Epidural hematomas have basically the same MRI signal characteristics as intracranial hematomas (see ▶ Table 4.2), though a number of factors may significantly influence the time course of signal changes in any given case:
The rising deoxyhemoglobin concentration, leading to decreased T2w signal intensity in the acute stage, is less pronounced with extra-axial hematomas (e.g., due to the higher oxygen tension in the extra-axial compartment and more difficult clot formation).
Arterial blood has a lower deoxyhemoglobin content (5%) than venous blood (40%). As a result, a high deoxyhemoglobin level is slower to develop in arterial hematomas, and the higher oxygen tension leads to a more rapid conversion of deoxyhemoglobin to methemaglobin than in venous hematomas.
Small hematomas have a greater surface area relative to volume. Consequently, the rate of clot formation and retraction and the formation of deoxyhemoglobin and methemaglobin vary with the size of the hematoma.
Dilution of the blood by cerebrospinal fluid (CSF), due for example to concomitant tears of the arachnoid, will reduce the concentration of paramagnetic substances, making the signal changes less pronounced.
Rebleeding may produce heterogeneous signal intensities (mixed light and dark areas).
Because acute extra-axial hematomas can be diagnosed most quickly and reliably by CT, enabling prompt referral for surgical decompression, no systematic studies have yet been published on their MRI signal characteristics in various stages. Nevertheless, findings in isolated cases confirm the principles summarized in ▶ Table 4.2. FLAIR sequences, by suppressing the signal from CSF, can facilitate the detection of small extra-axial hematomas ( ▶ Fig. 4.2).

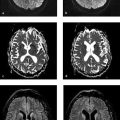
Fig. 4.2 Subacute subdural hematoma and hemorrhagic contusion. MRI examination 8 days after head trauma reveals a thin subdural hematoma over the right hemisphere. Thin extra-axial hematomas are much easier to detect by FLAIR imaging. In the T1w sequence, the hematoma is only slightly hyperintense to cortex. A small hemorrhagic contusion is also visible on the surface of the superior frontal gyrus. As we would expect at this stage, the contusion appears markedly hyperintense in both sequences. (a) Axial T2w FLAIR sequence. (b) Axial T1w TSE sequence.
Differential diagnosis Limitation of spread by cranial sutures is a more reliable differentiating criterion from subdural hematoma (see ▶ 4.4.3) than a biconvex shape. Generally the dura itself appears as a thin hypointense line on T2w or PDw images. Unlike subdural hematomas, almost all epidural hematomas occur on the side of the traumatizing impact (coup). Even so, it is not always possible to differentiate epidural hemorrhage from subdural hemorrhage. It is not unusual to find subdural blood deep to an epidural hematoma. Epidural hematomas over the posterior fossa, caused by dural sinus injuries, may exhibit simultaneous supratentorial and infratentorial spread.
4.4.2.2 Chronic Epidural Hematoma
MRI and CT findings Chronic epidural hematomas are rarely observed. They may exhibit a biconvex shape. They are hyperdense on CT and enhance after contrast injection. On MRI, they are predominantly hyperintense in both T1w and T2w images. Like any epidural mass, an epidural hematoma may cause medial displacement of cortical blood vessels and the dura. Chronic extra-axial hematomas have several distinctive MRI features:
They have less protein content than intracerebral hematomas due to constant dilution by CSF. As a result, they are not hyperintense on T1w images but are isointense or even slightly hypointense.
The increased T2w signal intensity is less pronounced than at the center of intracerebral hematomas or in subacute subdural hematomas.
Ferritin and hemosiderin deposits in an extra-axial hematoma may be removed by macrophages due to absence of a blood–brain barrier. They are found only on septa within the hematoma. Consequently, marked susceptibility artifacts are not seen in the hematoma or at its margins; at most, they may be detected on internal septa.
4.4.3 Subdural Hematoma
Epidemiology and pathogenesis Subdural hematomas are found in 10 to 20% of patients with head injury. They are the result of an acceleration–deceleration or rotational trauma mechanism. Most subdural hematomas result from injury to the bridging veins that run straight through the dura from the brain surface to the venous sinuses. These vessels are most vulnerable in their subdural segment. Direct sinus injuries (e.g., by penetrating trauma) may bleed on both the epidural and subdural sides. A large percentage of subdural hematomas occur in severe head trauma. The often severe associated intraparenchymal injuries (e.g., contusions or shearing injuries) contribute to the poor prognosis. Approximately 95% of subdural hematomas are supratentorial and 15% are bilateral. Most occur in the frontoparietal region.
4.4.3.1 Acute Subdural Hematoma
CT findings On CT, acute subdural hematomas are typically concave to the brain surface, uniformly hyperdense (60% of cases), or hyperdense with hypodense areas (40%). The hypodense areas represent either unclotted blood due to continued active bleeding or a CSF collection within the clotted hematoma due to arachnoid tears. Additionally, hypodense serum may be extruded during the early phase of clot formation. A biconvex shape is occasionally observed. Unlike epidural hematomas, however, the spread of subdural hematomas is not limited by sutural lines but by dural infolds (falx, tentorium). Very rarely, CT may show extra-axial hematomas that are not hyperdense but isodense to brain tissue. Possible causes are:
Physiologic hematoma resorption with decreasing density and a brief isodense stage in the second or third week.
Extremely low hematocrit (heavy blood loss with prehospital fluid replacement).
Coagulopathy.
Torn arachnoid under a subdural hematoma with mixing of blood and CSF.