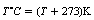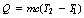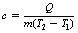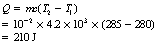Chapter 5 Heat
5.1 Aim
This chapter considers heat as a transfer of energy. Links between heat and temperature are established. The mechanisms of transferring heat are also established.
5.3 Heat energy and temperature
When heat is given to a body, its atoms or molecules are given increased kinetic energy in the form of increased lattice vibration (this is the reason why substances expand when heated). A body whose atoms have a higher kinetic energy than those of another body is said to be hotter or at a higher temperature. If two bodies are placed in contact, then heat will be transferred from the hotter body to the cooler body by collisions between the molecules at the point of contact. The molecules of the cooler body receive a net increase in kinetic energy and so its temperature rises. The molecules of the hotter body have lost kinetic energy and so its temperature falls. This process continues until the two bodies are at the same temperature when no further exchange of energy takes place. The bodies are now in a state of thermal equilibrium. Notice that the thermal energy is always transferred from the body at the higher temperature to the body at the lower temperature, irrespective of the size of the bodies. Also note that the temperature existing at thermal equilibrium will always lie somewhere between the initial temperatures of the two bodies.
5.3.2 Units of heat energy, specific heat capacity and thermal capacity
where Q is the heat energy required to raise the temperature of a body of mass m from T1 to a temperature T2. The factor c is approximately constant for a particular material and is known as the specific heat capacity of the material. We can rearrange Equation 5.2 to get:
so that c, the specific heat capacity, is in units of c joules per kilogram per kelvin (J.kg−1.K−1). Specific heat capacity can be defined thus:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree