Hemangioma, Lymphangioma, and Skin Cancer
Edward C. Halperin
L. Claude
John P. Kirkpatrick
HEMANGIOMAS
Hemangiomas are common developmental vascular abnormalities. These benign blood vessel tumors may be encountered in any portion of the body (1, 2, 3, 4, 5, 6, 7). In this chapter, we consider cutaneous, ocular, and vertebral body hemangiomas. Subglottic hemangiomas are discussed in Chapter 16.
Historically, hemangiomas were divided into three clinical types. Nevus flammeus (port-wine stains) is usually located on the head and neck, varying in size and color. Sturge-Weber syndrome (SWS) is a neurocutaneous syndrome consisting of port-wine stain in the cutaneous distribution of cranial nerve V, congenital glaucoma, and leptomeningeal angiomas (8,9). Nevus vasculosus (strawberry mark) is red and elevated. Angiocavernosum (cavernous hemangioma) was considered a lesion of the deep vasculature, especially the veins. Kasabach-Merritt phenomenon (KMP) is a rare thrombocytopenic consumption coagulopathy associated with an enlarging cavernous hemangioma or Kaposiform hemangioendothelioma (10). It may be complicated by a life-threatening situation due to thrombocytopenia, hemolytic anemia, and consumptive coagulopathy caused by platelet sequestration and high shear stresses within the lesion (Kasabach-Merritt syndrome) (10,11).
Modern classification divides vascular birthmarks into two broad categories—vascular tumors and vascular malformations—based on vasculogenesis, histopathology, and clinical features (6,12, 13, 14). Vascular tumors are dynamic lesions characterized by a growth phase, marked by endothelial proliferation and hypercellularity, followed by involution (6,12, 13, 14). This category includes the common hemangioma of infancy and rarer entities such as Kaposiform hemangioendothelioma, which is associated with Kasabach-Merritt syndrome (15,16). In contrast, vascular malformations are essentially stable, demonstrating little or no growth over time. These structural malformations include capillary (port-wine stain), venous, lymphatic, and arteriovenous malformations (Table 18.1). Hemangiomas often can be diagnosed by a thorough history and physical examination. If the natural history or appearance suggests a more aggressive neoplasm, imaging studies may prove useful in establishing a diagnosis. Doppler ultrasonography offers a rapid and inexpensive method of differentiating hemangiomas from vascular malformations (17). Tumor-specific vascularization pattern in contrast-enhanced ultrasound has a high diagnostic impact for the differential diagnosis of tumors in clinical practice, especially in hepatic localizations (18). Contrasted computed tomography (CT) or magnetic resonance imaging (MRI) scans provide detailed anatomic information of greater utility in planning therapy (17,19,20). The MRI appearance of a hemangioma differs according to evolutionary stage. During the proliferative phase, hemangiomas appear as well-circumscribed lobulated lesions that are hypo- to isointense relative to muscle on T1-weighted images, are hyperintense on T2-weighted images, and demonstrate early and homogeneous enhancement after gadolinium-based contrast material administration (21,22). During the involuting phase, as increasing amounts of fat replace the tumor, foci of increased signal intensity are noted within the lesion on T1-weighted images, associated with less avid contrast enhancement (22,23). The choice of imaging modality depends on the specific anatomic location of the lesion. For example, contrasted T1-weighted and T2-weighted MRI are particularly effective at distinguishing vascular tumors of the head and neck from other soft tissue structures. MRI has also been well described and has shown an important role in the detection and diagnosis of pure spinal epidural cavernous hemangioma. It shows a characteristic lobulated contour, which encircled the spinal cord. T1-weighted images on the MRI presented as isointense and T2-weighted images presented as hyperintense and a homogeneously
strong enhancement (24). The differential diagnosis for these vascular tumors, apart from vascular malformations, includes mainly infantile fibrosarcoma and dermatofibrosarcoma protuberans (25).
strong enhancement (24). The differential diagnosis for these vascular tumors, apart from vascular malformations, includes mainly infantile fibrosarcoma and dermatofibrosarcoma protuberans (25).
Table 18.1 Classification of Congenital Vascular Abnormalities | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Only 20% of hemangiomas are present at birth. The other 80% arise within the first 8 weeks of life. The natural history of the common hemangioma of infancy is one of the rapid growth during the first 6-9 months of life and then a period of more slowly increasing size that parallels the infant’s growth. Involution generally follows at a rate of about 10% per year, with complete disappearance of 50% of the lesions by 4 or 5 years of age and 90% by 9 years (6,14,26). Hemangiomas are most commonly seen on the face and neck (60% of cases), followed by the trunk (25%) and extremities (15%) (27), and are multiple in about 20% of cases (15). At pathologic analysis, common hemangiomas of infancy express a unique immunophenotype known as glucose transporter protein-1, which is not seen in vascular malformations, other vascular tumors, or the vasculature of malignant tumors (28). In most cases, hemangiomas of infancy are best managed by observation (29). A prospective, randomized controlled trial on 121 infants aged 1-14 weeks with early hemangiomas was recently published (30). The aim was to compare treatment with pulsed dye lasers with a wait-and-see policy. The number of children whose lesions showed complete clearance or minimum residual signs at 1 year was not significantly different in the treated and observation groups (42% vs. 44%; p = 0.92).
However, there are some indications for treatment, including rapid progression of the lesion producing unacceptable symptoms (e.g., an eyelid hemangioma, obstructing vision and producing deprivation amblyopia); progressive ulceration and infection (perineal lesions are at particular risk); progression of a lesion causing unacceptable deformity from compression, or overgrowth of an extremity from increased blood flow in a hemangioma (hemangiomatous gigantism); growth of a lesion in an intertriginous area, where it is subject to trauma and secondary infection; facial lesions producing severe cosmetic deformity; high-output cardiac failure; and life-threatening Kasabach-Merritt syndrome (3,5, 6, 7,29, 31, 32, 33, 34). When the decision is made to treat a hemangioma, options available include topical, intralesional, and systemic steroids in first-line approach (29). In the treatment of hemangiomas, 30-80% of lesions respond to steroids (2,6,35). In second line, interferon, laser therapy, and surgical resection are the options. Interferon is often effective, although there have been reports of severe neurotoxicity, including spastic diplegia (36, 37, 38, 39). Embolization, vasoligation, chemotherapy (cyclophosphamide, vincristine …), and angiogenesis inhibitors can also be discussed (6,31, 32, 33, 34, 35, 36,38,40, 41, 42, 43, 44). Resection or arterial ligation is indicated for small lesions for which excision would produce significant cosmetic deformity. Surgery is also appropriate for treatment of uncontrolled ulceration, bleeding or infection, arteriovenous shunts with high-output cardiac failure, or visual obstruction (3,5, 6, 7).
Radiotherapy should be considered only for lesions that have failed to respond to steroids or interferon and are not appropriate for treatment with other modalities. Significant late effects have been attributed to irradiation of hemangiomas, which include scarring, bone growth abnormalities with dental and periodontal long-term effects (42,45), and secondary neoplasms (33,46, 47, 48, 49, 50, 51). The incidence of cancer after radiotherapy for skin hemangiomas has been studied extensively in a series of articles from Sweden. These reports describe a total of 14,633 children below 18 months irradiated at the Radiumhemmet in Stockholm from 1909 to 1959 and 12,055 treated at Sahlrenska Hospital, Goteborg, from 1930 to 1965 (46, 47, 48, 49, 50, 51). At the Radiumhemmet, treatment was most commonly by 226Ra applicators (81%) or contact x-ray therapy (60 kVp or less, 16%). At Goteborg, 226Ra was used for 99% of the treatments. The median age of treatment was 6 months at Radiumhemmet and 5 months at Goteborg. There is a higher-than-expected incidence of cancer in these irradiated children. Significantly higher levels of breast cancer were detected in the pooled studies (hazard ratio 1.2, 95% confidence interval (CI): 1.06-1.36), and there was an approximate twofold increase in the expected incidence thyroid cancer. Recalculation of the dosages from the 226Ra applicators suggests a linear dose-response relationship. Conceivably, the use of electron therapy in lieu of 226Ra might mitigate the long-term effects of radiotherapy in these patients. Another large French report on 8307 patients treated for 4767 hemangiomas was included in an incidence study, among whom 3795 had received radiotherapy (52). External radiotherapy, radium 226, strontium 90, yttrium 90, and phosphorus 32 were used. The mean estimated radiation dose received by the thyroid during radiotherapy was 41 mGy. This study confirms that radiation treatment performed in the past for hemangioma during infancy increased the risk of thyroid carcinoma and adenoma. A significant dose-response relationship was found between the radiation dose received by thyroid and the risk of thyroid cancer (excess relative risk per GY, ERR/Gy: 14.7, 95% CI: 1.6-62.9) These studies strongly argue against the routine use of radiotherapy to treat hemangiomas of infancy and indicate that irradiation of the thyroid, breast buds, and gonads should be avoided (46, 47, 48, 49, 50, 51, 52). The response of cutaneous hemangiomas of infancy to irradiation can be dramatic. In 1940, Kasabach and Merritt originally reported a combination of external irradiation with superficial x-rays and interstitial irradiation with radium needles as a successful primary treatment (53). Furst et al. reported good results in 88% of treated cases (41,48). In treating patients with Kasabach-Merritt syndrome, the objectives are both regression of the hemangioma and control of the thrombocytopenia and coagulopathy. Ogino et al. demonstrated regression of the lesion and an increase in platelet count to more than 105/mm3 within 40 days of radiotherapy (43). The rise in platelet count appears to parallel corresponding clinical evaluations. It has been suggested that a platelet count response may predict tumor regression following radiotherapy (54). When radiotherapy is used, gross tumor volumes should ideally be defined clinically, with the aid of CT or MRI to determine the optimal field sizes (32). Minimal margins should be used to yield the least morbidity. Fraction sizes vary from 1 to 2.5 Gy and schedules commonly apply daily treatments (43). The majority of lesions respond to two or three fractions for a total dosage of 300-750 cGy. Occasionally, higher dosages may be indicated, but the total dosage should not exceed 10 Gy (43,54). If these guidelines are observed, regressions generally will occur rapidly, and scarring should be minimal.
On rare occasions, the radiation oncologist is consulted on cases of hemangiomas that began in infancy and continued
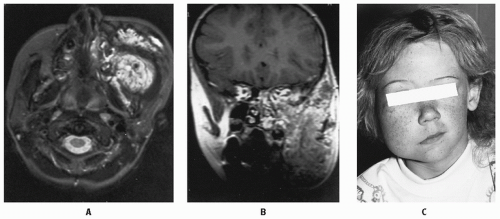
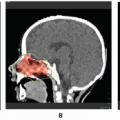
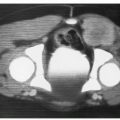
to enlarge slowly rather than regress. These older children or young adults present with severe deformity and rarely hemodynamic compromise, anemia, or coagulopathy (55). A host of other treatments fail before radiotherapy is considered for these patients. The response of these large, symptomatic lesions often is substantial (32,34,56). Before treatment, the lesion should be evaluated by careful physical examination and by imaging, preferably by MRI as described earlier. The lesion is irradiated with tight margins using fractionated radiotherapy in 150-200 cGy daily fractions, typically to a total dosage of 30-40 Gy (56). (See Figure 18.1)
to enlarge slowly rather than regress. These older children or young adults present with severe deformity and rarely hemodynamic compromise, anemia, or coagulopathy (55). A host of other treatments fail before radiotherapy is considered for these patients. The response of these large, symptomatic lesions often is substantial (32,34,56). Before treatment, the lesion should be evaluated by careful physical examination and by imaging, preferably by MRI as described earlier. The lesion is irradiated with tight margins using fractionated radiotherapy in 150-200 cGy daily fractions, typically to a total dosage of 30-40 Gy (56). (See Figure 18.1)
Orbital hemangiomas are another special treatment case (57,58). When irradiation of the lid is needed, the eye is protected by a lead shield placed behind the lid and over the lens of the eye. Superficial x-rays or electrons are then used for treatment. Posterior orbit hemangiomas may be successfully treated to a total dosage of 12-18 Gy using techniques similar to the commonly used lateral beam approach for retinoblastoma (see Chapter 5) (32). Gamma knife radiosurgery may be an effective treatment option for selected orbital hemangiomas, while increasing the possibility of visual function preservation in selected cases in which the lesion is adjacent to the optic apparatus (59).
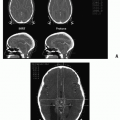
Hemangiomas of the bone are benign, slow-growing tumors that can exhibit cavernous or capillary architecture. The majority of these lesions are asymptomatic and are detected on routine radiographs. The hemangioma produces replacement of the normal cancellous bone with thick bony trabeculae. On plain radiographs and CT scan, a honeycomb or polka-dot appearance is characteristic (60). The incidence increases with age, and less than 10% occur in the first two decades of life (61). Hemangiomas of the vertebrae may give rise to neurologic signs and symptoms ranging from pain to severe spinal cord compression. The pathophysiology of these lesions includes ballooning of the vertebrae with narrowing and deformation of the spinal canal, extension of the hemangioma into the epidural space, and, rarely, vertebral body compression fracture. Although asymptomatic vertebral bodies do not warrant treatment, intervention is needed for paresis, paraplegia, or severe pain. Laminectomy is recommended by some in the setting of rapid-onset cord compression but is hazardous because of the chance of severe hemorrhage. Catheter embolization of feeder vessels has been successful but runs the risk of vascular catastrophe (60).
Rades et al. (62) analyzed the results from University Hospital Eppendorf and published reports on the outcome after radiation therapy in the treatment of symptomatic vertebral hemangiomas (60, 61, 62, 63, 64, 65). A total of 117 patients were evaluable, ranging in age from 12 to 78 years (median age 47 years). To identify a dose-effect relationship, patients were divided into two groups based on the total radiation dosage of 20 Gy (n = 62, median total dose 30 Gy) or 36 Gy (n = 55, median dose 40 Gy). Total radiation dosages were calculated based on a 2-Gy per fraction equivalent dosage using a ratio of 3 Gy. Complete pain relief was achieved in 82% of the higher dose group and 39% of the lower dose group (p = 0.003). Complete or partial pain relief was obtained in 100% and 87% of the higher and lower dose patients, respectively. This analysis suggests that a total of 40 Gy delivered in 2 Gy per fraction can provide effective palliation of symptomatic vertebral hemangiomas (62).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree