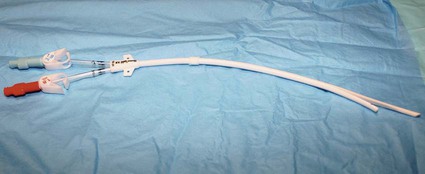
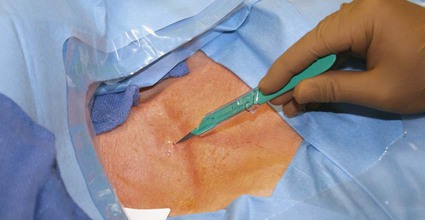
In the United States, the number of patients with end-stage renal disease (ESRD) has been rising each year (often by > 10%), and more than 300,000 individuals undergo hemodialysis treatment.1 Each of these patients needs some form of vascular access to allow sufficient blood flow to the dialyzer for adequate hemodialysis, which is administered three times weekly. Maintaining vascular access patency and function for these patients is an essential component of their care. The Clinical Practice Guidelines and Recommendations of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) state that an ideal vascular access should have the following characteristics: optimum flow rate for adequate hemodialysis, long use-life, and low rate of complications (e.g., infection, stenosis, thrombosis, aneurysm, and limb ischemia).2 Although no currently available vascular access can perfectly meet these criteria, the surgically created native arteriovenous fistula (AVF) has been reported to have the best patency rates, require the fewest interventions, and have the best results following interventions compared to other types of vascular access.3–5 Despite the NKF KDOQI Vascular Access Guidelines that recommend increased placement of AVFs and decreased use of hemodialysis catheters, 10% of total vascular accesses, the patient population with ESRD receiving hemodialysis via catheters has steadily increased from 13% to 25%. This increase of hemodialysis catheters may be partly attributed to advanced patient age, comorbid conditions such as peripheral vascular disease and diabetes, and late referral for creation of an AVF.6 Hemodialysis catheters still play an important role, not only as a temporary access for patients with acute renal failure but also as a bridge until a more definitive form of vascular access is established. According to the guidelines and recommendations developed by the NKF KDOQI Vascular Access Work Group, one of the goals to improve patients’ survival and quality of life is to increase the placement of AVFs. Recently, the target for fistula creation has been reset at 65%.2 Despite NKF KDOQI recommendations, decreased durability, and a higher rate of complications with catheters, some patients may nonetheless prefer them to AVFs and grafts.7 One significant difference for the patient is the necessity for needle access to the fistula or graft at two separate skin sites during each dialysis session, versus painless direct connection of the dialysis catheter to the dialysis machine. The use of hemodialysis catheters as primary vascular access should be minimized because of the high risk for infection and high malfunction rate. The NKF KDOQI Vascular Access Work Group strongly recommends that catheters be used in less than 10% of hemodialysis patients as their permanent chronic hemodialysis access.2 The preferred site of catheter placement is the right internal jugular (RIJ) vein because of its more direct route to the right atrium than the left-sided venous approach. Catheter placement in the left internal jugular (LIJ) vein may potentially limit permanent access options on the left arm by inducing stenosis or occlusion of the left brachiocephalic vein. It has been reported that catheter placement in the LIJ vein may be associated with decreased blood flow rates and increased risk for stenosis and thrombosis.8,9 Catheter placement in the subclavian vein on either side should be avoided because of the risk for stenosis,10,11 which can significantly reduce the possibility of future upper extremity permanent AVF or graft. Catheters should not be placed in the vein on the same side as a slowly maturing permanent access. Central venous stenosis due to catheter placement is closely linked to the site of insertion,12,13 number and duration of catheter uses, and occurrence of infection.12,14 Although the common femoral vein may be used for nontunneled temporary access, longer-term access with tunneled catheters and ports is suboptimal and relatively contraindicated because of the higher rate of infection at this site.15 According to the duration of use, hemodialysis catheters can be divided into acute short-term nontunneled catheters and long-term tunneled cuffed catheters. Tunneled cuffed catheters and ports are considered as vascular accesses for hemodialysis over weeks to months.2 Nontunneled catheters are designed for temporary hemodialysis access, especially in patients who need acute renal replacement therapy (Fig. e121-1). Although these catheters are ready for immediate use, they have a limited use-life and therefore should not be placed until they are absolutely needed. It is recommended that IJ nontunneled catheters should not be used for more than 1 week because of an infection risk.16–18 These catheters directly enter a central vein, with no intervening tunnel beyond the skin entry site, so infection is a greater risk over the long term. After 1 week, the infection rate increases exponentially.19 The femoral vein may occasionally provide an alternative access route when necessary, but it should be used for no more than 5 days because of the increased risk for infection.15 The tip of the femoral nontunneled catheter should be positioned in the inferior vena cava to reduce recirculation.20 A patient with a nontunneled hemodialysis catheter should not be discharged while awaiting the next hemodialysis, owing to the risk for infection, inadvertent dislodgement, hemorrhage, air embolism, and discomfort. Before discharge, a short-term nontunneled catheter should be exchanged for a tunneled cuffed catheter unless there is active infection.21 Tunneled catheters have a Dacron cuff around a portion of the catheter that will sit within the subcutaneous tunnel. Ingrowth of subcutaneous tissue into the cuff makes it adherent to the tunnel and “seals” the tunnel. This is thought to help prevent migration of bacteria from the skin surface along the catheter and into the bloodstream, thereby guarding against infection.22 The cuff also helps retain the catheter and thus prevents dislodgement and malpositioning (Fig. e121-2). Unlike infusion catheters, to be effective, hemodialysis catheters must have two different locations where the two lumina communicate with the bloodstream. In this way, blood can be collected upstream and then returned after passage through the hemodialysis machine in a relatively downstream location. This prevents the catheter from recollecting blood that has just passed through the dialysis machine and reentered the patient, an undesirable mixing phenomenon known as recirculation, which will decrease the efficiency of hemodialysis.23 Catheters vary somewhat in their diameter, with most falling between 11F and 16F. Differences in diameter and end-hole/sidehole arrangement may have some influence on flow rates catheters are capable of achieving. Catheters should be able to deliver a consistent flow greater than 350 mL/min to the hemodialysis machine at prepump pressures of −200 to −250 mmHg. The choice for the best device should be made based on comparison of flow data, local experiences, goals for use, and cost.2 Fibrin sheath formation around the distal portions of hemodialysis catheters has been associated with catheter malfunction and poor flow. In an effort to reduce this problem, some catheter systems have been designed with a split tip (Ash-Split catheter [Medcomp]). In other words, the distal portion of the catheter splits into two separate lumina, just as the proximal external portion of the catheter is divided into two separate lumina. It has been suggested that motion of the two lumina within the bloodstream during the cardiac cycle, including motion of one lumen against the other, will help inhibit the formation of fibrin sheaths.24 Catheters with a split-tip design may also minimize recirculation effect.25,26 Subcutaneous implantable port systems have been developed in an effort to reduce the rate of infectious complications.27–30 The LifeSite hemodialysis port consists of two individual metallic subcutaneous reservoirs, each of which attaches to a 12F catheter with multiple side holes. They are accessed with standard 14-gauge access needles of the type that would be used to access a fistula or graft. The ports automatically lock the needles in place when they are turned.31 The Dialock Access Port is a rectangular septumless device with a titanium shell and two internal valves. On the anterior and inferior portion of the device, there is a shelf with two grooves that are used for two 15-gauge access needles into the tubular chambers. The access needle is locked after passing the valve in the device. Each chamber is connected to an 11F silicone catheter with notched distal end hole.30 • Review of the patient’s history and relevant radiologic images • Using maximum sterile barrier technique for preparation and during the entire procedure • Ultrasound-guided venous access, with caution to avoid inadvertent injury to the carotid artery • Measuring the length from the neck venotomy site to the right proximal atrium • Creation of tunnel from below the clavicle, with gentle curve to avoid a kink in the catheter • Placement of cuff in the tunnel 1 to 2 cm from the tunnel exit site at the chest • Closing the skin incision sites and securing the catheter with sutures • Filling each lumen of the catheter using optimal volume of 5000 units/mL of heparin • Closing the hub of each lumen of catheter using specialized cap Limited ultrasound examination of the planned access site is very useful for quick assessment of the feasibility of an approach. Strict sterile technique should be used, including sterile preparation and draping of the patient. Currently in most institutions, skin is prepared using 2% chlorhexidine gluconate in 70% isopropyl alcohol (Chloraprep).32 The skin surface should be shaved before sterile preparation if hair is visible. For tunneled catheters placed via the IJ access site, preparation should extend from the upper mid-neck area to the nipple. Full surgical scrubbing is a necessity for all operators, as is the use of surgical caps, masks, and sterile gowns and gloves. Sterile probe covers should be used for ultrasound probes. Preprocedure antibiotic administration is not required,33,34 with multiple studies demonstrating low infection rates that are comparable or lower than those documented for surgical placement.35 Because the IJ vein is the preferred hemodialysis catheter placement site, discussion will focus on this approach. Real-time ultrasound guidance should be used for IJ venous puncture because it will help guard against complications. The IJ vein should be accessed in the inferior aspect of the neck as close to the superior border of the clavicle as possible. A low puncture will aid in preventing kinking of the catheter as it curves from the subcutaneous tunnel to the venotomy site. After ultrasound is used to select the access site, local anesthesia should be administered. A small, shallow incision or “skin nick” should be made over the access site with a #11 blade (Fig. e121-3 Fluoroscopy is used to position the wire tip in the upper to mid–right atrium. The wire is then measured to determine the appropriate catheter length for the patient. Two methods of measurement are commonly used, depending on the preference of the operator. One involves marking the wire at the point where it exits the transition dilator (Fig. 121-3). Then the intravenous portion of the wire can be measured by subtracting the length of the exposed transition dilator from the length of the wire between the tip and the marking. This is the best way to measure the appropriate length for a nontunneled catheter, and many operators also use this measurement to determine the length of a tunneled catheter. However, when placing tunneled catheters, some operators prefer to bend the wire in a curved pathway that simulates the course of the catheter through the tunnel, and mark the wire at a reproducible point intended to be at or near the exit site of the tunnel, such as three fingerbreadths below the clavicle (Fig. e121-4
Hemodialysis Access
Catheters and Ports
Introduction
Indications
Contraindications
Equipment
Nontunneled Catheter
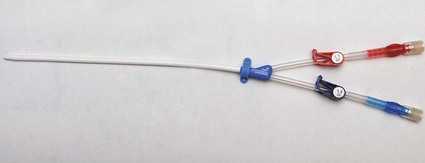
Tunneled Cuffed Catheter
Port
Technique
Anatomy and Approach
Technical Aspects
![]() ). Use of a hemostat to spread the underlying fascia will facilitate subsequent passage of the peel-away sheath and also aid in preventing catheter kinking at the venotomy site. Through the skin incision, the IJ vein is accessed using a 21-gauge needle connected to a 20-mL syringe by a short connecting tubing under ultrasound guidance. Placement of the needle tip within the vein is confirmed by ultrasound and aspiration of blood through the 20-mL syringe (Fig. 121-1). Under fluoroscopic guidance, a 0.018-inch guidewire is advanced through the needle and subsequently exchanged for a Cope transition dilator (Cook Medical, Bloomington, Ind. [Fig. 121-2]). Some operators prefer an 18-gauge needle for venous access to skip the transition from 0.018-inch to 0.035-inch systems. Others prefer a micropuncture set (Cook Medical) to make the transition to a 0.035-inch system.
). Use of a hemostat to spread the underlying fascia will facilitate subsequent passage of the peel-away sheath and also aid in preventing catheter kinking at the venotomy site. Through the skin incision, the IJ vein is accessed using a 21-gauge needle connected to a 20-mL syringe by a short connecting tubing under ultrasound guidance. Placement of the needle tip within the vein is confirmed by ultrasound and aspiration of blood through the 20-mL syringe (Fig. 121-1). Under fluoroscopic guidance, a 0.018-inch guidewire is advanced through the needle and subsequently exchanged for a Cope transition dilator (Cook Medical, Bloomington, Ind. [Fig. 121-2]). Some operators prefer an 18-gauge needle for venous access to skip the transition from 0.018-inch to 0.035-inch systems. Others prefer a micropuncture set (Cook Medical) to make the transition to a 0.035-inch system.
![]() ). The distance from this mark to the tip of the wire is the length from the tunnel exit site to the tip of the intravenous portion of the catheter. The wire is subsequently removed from the transition dilator.
). The distance from this mark to the tip of the wire is the length from the tunnel exit site to the tip of the intravenous portion of the catheter. The wire is subsequently removed from the transition dilator.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Hemodialysis Access: Catheters and Ports