36
Hemodialysis Access Management
Dialysis is defined as the removal of blood elements by diffusion through a semipermeable membrane. The purpose of dialysis is to remove metabolic waste products and maintain fluid and electrolyte balance. The hemodialysis machine functions as an “artificial kidney.” Blood is drawn from the patient at a rate of 300 to 500 mL per minute, passed through the dialysis machine, and then returned to the patient. Patients with end-stage renal disease typically require hemodialysis three times per week for 3 to 4 hours. The rapid circulation of blood between patient and hemodialysis machine requires a conduit that is durable, has a low infection rate, and is easily accessible. Peritoneal dialysis is an alternative form of dialysis in which dialysate is instilled into the peritoneal cavity, periodically drained, and replaced with fresh solution via a catheter. The peritoneal membrane acts as the dialyzing surface in peritoneal dialysis.
Alternatives for access to the patient’s blood circulation for hemodialysis include the use of a central venous catheter, a surgically created arteriovenous fistula, or a prosthetic graft interposed between an artery and vein. Central venous catheters have one lumen for drawing blood from the patient into the hemodialysis machine (arterial ) and one lumen for blood return to the patient from the hemodialysis machine (venous ). Access to a prosthetic graft or the venous outflow of a surgically created fistula is made with two 14- to 16-gauge needles.
Renal transplantation is the treatment of choice for many patients with end-stage-renal disease. Because of limited organ availability, however, dialysis is the primary therapy for most of these patients. More than 280,000 persons in the United States require chronic dialysis.1 For these patients, functioning dialysis access is their “lifeline,” and maintenance of this access is critical. Hemodialysis access maintenance represents one of the most challenging problems for interventional radiologists. Thrombosis is the most common cause of hemodialysis access graft loss and usually is related to stenoses in the venous outflow. The goal of hemodialysis access maintenance is to detect and treat access dysfunction prior to access thrombosis and to salvage thrombosed grafts.
 Arteriovenous Graft
Arteriovenous Graft
Most patients requiring chronic hemodialysis have an upper-extremity arteriovenous graft (AVG) constructed of 6-mm-diameter polytetrafluoroethylene (PTFE) tubular graft material. Typical upper-extremity grafts include a loop graft with anastomoses at the brachial artery and basilic vein or cephalic vein and a straight graft with anastomoses at the distal radial artery and the basilic or cephalic vein near the antecubital fossa. The graft is tunneled subcutaneously and is easily accessible by percutaneous needle puncture. A lower-extremity loop graft extending from the common or superficial femoral artery to the saphenous vein is constructed when upper-extremity veins are no longer usable for graft construction. The most common complication associated with PTFE hemodialysis access grafts is the development of stenosis at the venous anastomosis or venous outflow of the graft resulting in graft thrombosis. Patency rates at 1 year range from 55 to 75%,2,4,5 and the life span of the average graft is estimated to be less than 2 years.6
 Brescia–Cimino Fistula
Brescia–Cimino Fistula
The preferred access for hemodialysis is an endogenous arteriovenous fistula because it provides the greatest chance for long-term function. A Brescia–Cimino arteriovenous fistula is usually constructed with a side-to-side anastomosis of the cephalic vein and radial artery at the wrist. A fistula also can be placed at the antecubital fossa with an anastomosis of the brachial artery and cephalic vein. Brescia–Cimino fistulas are less likely to thrombose than a PTFE graft because a fistula has a single anastomosis with multiple potential areas of venous outflow.
After an arteriovenous fistula is created, the patient cannot undergo dialysis using this access until fistula maturation occurs. Maturation involves vein-wall thickening and dilatation, which facilitates vein puncture with large-bore needles and accommodates arterial blood flow required for dialysis. The maturation process typically takes 1 to 4 months. Fistulas are ideally placed several months prior to anticipated use to ensure adequate maturation.
Unfortunately, Brescia–Cimino fistulas have a high early failure rate, usually as a result of inadequate venous size or runoff. When the fistula is established and used successfully for hemodialysis, late failure is rare. In about 20 to 30% of cases, the fistula will never mature.2–4
 Causes of Graft Dysfunction
Causes of Graft Dysfunction
Thrombosis is the most common cause of hemodialysis access graft loss and usually is related to stenoses in the venous outflow. If thrombosis occurs in the first few weeks after placing the access, there is often a technical problem related to the graft. Technical problems include graft kinking, narrow arterial or venous anastomoses, or preexisting arterial inflow or venous outflow disease (Fig. 36-1).7 Occasionally, there is rapid development of venous outflow stenosis after graft insertion.8 Thrombosis that occurs after 2 to 3 months is usually due to the development of stenosis in the venous outflow. Arterial inflow stenosis may occur, but it is a much less frequent problem.9 Occasionally, no underlying anatomic lesion can be found. It has been postulated that these grafts may have thrombosed due to excessive postdialysis graft compression at the needle puncture sites, hypotension, hypovolemia, compression of the graft due to sleeping position, or a hypercoagulable state.10
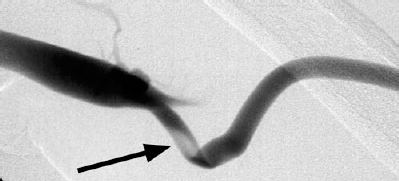
FIGURE 36-1. Kinking of this newly placed hemodialysis graft near the venous anastomosis (arrow) required surgical revision.
The development of venous stenoses appears to be caused by neointimal hyperplasia. The cause of this hyperplasia is not well understood. A variety of theories have been proposed to explain the development of neointimal hyperplasia. One mechanism of hyperplasia is a response to turbulent blood flow and vibration resulting from placement of the graft.11 Other factors such as the transition between the relatively noncompliant graft material and the compliant vein,12 or angulation and stretching of the vein at the anastomosis,13 may be important. Whatever the cause, these stenoses inevitably recur after intervention because the underlying pathophysiology is unchanged. This is the cause for the ongoing requirement for repeated angioplasty and thrombolysis of the dialysis grafts. Until the pathophysiology of intimal hyperplasia is understood and techniques are developed to prevent intimal hyperplasia, there will be a need for the mechanical treatment of venous stenoses.
 Treatment of the Poorly Functioning Graft
Treatment of the Poorly Functioning Graft
The sites available for hemodialysis access are limited; therefore, it is important to extend the life of each access for as long as possible. Treatment of grafts prior to thrombosis is more cost- and time-effective than treatment of thrombosed grafts. Long-term patency of grafts is improved if stenoses are treated prior to thrombosis as opposed to undertaking angioplasty or surgical revision after thrombosis has occurred; however, this requires identifying functioning grafts that are at risk for thrombosis. If the developing stenosis can be identified and treated with angioplasty, the risk and cost of thrombolysis can be avoided.
A number of signs may identify developing graft stenoses, including elevated venous pressure,8,14 difficulty with needle placement, increased bleeding following needle removal, extremity edema, dilatation of collateral veins over the upper extremity or chest,15 and conversion of the normal graft thrill to a pulse.16,17 Venous pressure refers to the pressure measured by the dialysis machine as blood is returned to the patient. Venous return pressure greater than 150 mm Hg at a blood flow of 200 mL per minute is indicative of a venous outflow stenosis.18 Recent studies showed that Doppler ultrasound may be useful in the evaluation for stenoses.19,20
Angiographic evaluation of a hemodialysis access graft should include the arterial anastomosis, graft, venous anastomosis, and the entire venous outflow. This can be done easily by using a dialysis needle, which can be left in place following a dialysis session or using standard Seldinger technique. If a stenosis is identified, angioplasty can be performed immediately and the patient discharged following completion of the procedure (Fig. 36-2).
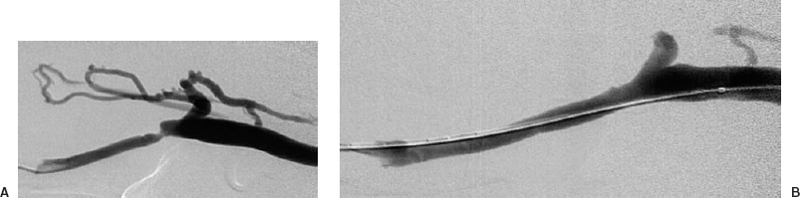
FIGURE 36-2. Arteriovenous shunt stenosis. Venous anastomotic stenosis. (A). Before the 7-mm balloon angioplasty. (B). After angioplasty.
Early correction of venous stenoses prolongs access viability. A recent study followed up on 106 grafts suspected on clinical examination to have a venous stenosis.21 The grafts were studied angiographically, and any venous stenosis was treated with balloon angioplasty. The technical success for angioplasty was 98%. The primary patency at 1 year was 23% for PTFE grafts. Repeated angioplasty improved the patency rate to 68% at 1 year and 51% at 2 years (primary assisted patency rate). A combination of thrombolysis and repeated angioplasty improved patency further, with secondary patency rates at 1 year of 82% and at 2 years of 65%.21
Recurrence of stenoses should not be considered a failure of the procedure but rather an expression of the underlying pathophysiology. Redilatations allow easy and safe maintenance for months and even years.17 Gaining added months of patency through redilatations could be of great value for dialysis patients. In contrast to a surgical graft revision, loss of central veins does not occur with angioplasty. Because angioplasty and surgical techniques may have similar secondary patency, the loss of future access sites becomes an important issue.
The treatment of a poorly functioning Brescia–Cimino fistula is less likely to involve thrombolysis and more likely to require angioplasty of a venous stenosis. In patients with Brescia–Cimino fistulas, most stenoses occur in the anastomotic and postanastomotic area before the puncture area for dialysis.22 These fistulas are difficult to evaluate because of the number of outflow veins that become opacified with angiographic evaluation. This is particularly apparent when a stenosis in the main outflow and development of collateral veins occur. The collateral veins may overlie and obscure the venous stenosis, and angiography in multiple projections may be required to demonstrate an abnormality.
 The Thrombosed Graft
The Thrombosed Graft
The traditional therapy for failing hemodialysis access has been surgical thrombectomy with or without revision of the venous anastomosis. Thrombectomy alone is usually not successful because thrombectomy does not address the cause of graft failure, and replacement is not a realistic option because the possible access sites would be exhausted rapidly.
During the past 10 to 15 years, percutaneous techniques have become widely used for dialysis access salvage. Graft thrombolysis and balloon angioplasty of stenoses are the essence of percutaneous therapy for clotted hemodialysis access grafts. The thrombolysis portion of the procedure can be performed by several methods, including a combination of clot-dissolving medications and mechanical “declotting” devices.
A hemodialysis graft has unique characteristics that make it particularly amenable to percutaneous therapy. The graft is easy to access percutaneously, contains fresh clot responsive to thrombolytic agents, and is a closed system with only a single inflow and outflow, keeping the thrombolytic agent from diffusing into the systemic circulation.
The percutaneous approach to a thrombosed dialysis graft allows not only for thrombolysis but also for angiographic evaluation of the graft. The entire graft, including the arterial inflow and the complete venous outflow, can be evaluated easily. The venous anastomotic stenosis that is often encountered can be treated with balloon angioplasty.
The percutaneous graft declotting and angioplasty procedure is performed in a single session and usually is performed on an outpatient basis. Following the conclusion of the procedure, the patient can be discharged to a dialysis unit or home. The graft is functional for dialysis immediately following percutaneous therapy, unlike after surgical revision or new graft insertion. This is crucial because successful percutaneous therapy obviates the need for the insertion of a temporary central venous hemodialysis catheter. Avoidance of the use of temporary jugular or femoral central venous dialysis catheters decreases expenses and eliminates the risk of complications from these catheters, such as central venous stenosis and infection.
 Patient Selection
Patient Selection
Usually, thrombosis of a recently (within a few weeks) constructed graft is due to a surgical technical problem. Sometimes thrombolysis is performed to identify the cause of the problem, which may allow a focused surgical repair. There is some increased risk of bleeding from recent anastomotic sites; however, if this does occur, it is usually controlled easily with manual pressure. Balloon angioplasty of the anastomosis of a recently placed graft, however, is at increased risk for rupture and should be avoided.
Some patients should not undergo thrombolysis. Criteria that would exclude a patient with a thrombosed dialysis graft are the same for other thrombolytic procedures. Absolute contraindications to thrombolysis are active gastrointestinal bleeding and recent neurologic processes, including intracranial bleeding, stroke, or neurologic surgery. Relative contraindications include major surgery or organ biopsy within 2 weeks; recent serious trauma; preexisting coagulation defects; uncontrolled, severe hypertension; pregnancy or the immediate postpartum period. The use of a mechanical thrombectomy device may be ideal for patients at increased risk for bleeding.
A contraindication to percutaneous graft thrombolysis is the presence of a graft infection. Lysis of infected clot may precipitate bacteremia and lethal sepsis.23 Also an infected clot is relatively resistant to thrombolysis. Determining the presence of infection in a graft is often difficult. The classic signs of infection, such as redness, tenderness, warmth, swelling, and fever, are often blunted in patients with uremia. Purulent discharge or skin breakdown around the graft are obvious signs of infection, but these sign are uncommon. Needle aspiration of the graft clot for Gram stain and culture or of perigraft fluid collections found on ultrasound evaluation may be helpful in diagnosing graft infections.24 Infection of the graft mandates treatment with antibiotics and referral for surgical removal of the graft.
Grafts that have undergone attempts at access just prior to the thrombolytic procedure are more likely to develop bleeding from the puncture sites during the thrombolytic procedure. It is worthwhile to develop a policy with the referring dialysis center so that if a patient presents with a pulseless graft, no attempts will be made to cannulate the graft, and the patient is immediately referred for percutaneous recanalization. Bleeding occasionally develops from puncture sites that are several days old, particularly if the puncture was traumatic. Usually bleeding is not a serious problem. Bleeding can be managed by manual compression over the site and by rapid relief of the outflow stenosis by angioplasty that will decrease the pressure within the graft. On occasion, it is not possible to control the bleeding site while continuing to recanalize the graft. In this situation, surgical therapy may be required.
 Graft Recanalization Technique
Graft Recanalization Technique
Graft access
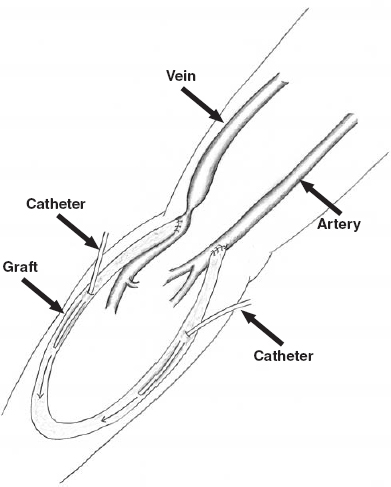
The crossed-catheter technique is the basis of the approach to the thrombosed graft (Fig. 36-3 and Table 36-1).25–27 This approach allows access to both the arterial and venous ends of the graft simultaneously. A standard single-wall entry needle or a Micropuncture Introducer set (Cook, Bloomington, IN, U.S.A.) is used to access the graft. The first puncture is made in the graft at the junction of the proximal and middle third of the graft (the arterial end of the graft) directed toward the venous end of the graft. The graft can be accessed most successfully if it is fixed between the thumb and index finger and held firmly while the puncture is being made. As the graft wall is punctured, there is a “popping” sensation. This sensation can be blunted in an older graft because scar tissue builds up around the graft after many punctures. The graft is thrombosed; thus, there will be no pulse within the graft and usually no return of blood when the lumen is entered. When the graft is entered, the guidewire usually passes easily through the lumen, even in the presence of clot. In some cases, a small amount of dark blood returns through the needle.
FIGURE 36-3. Initial catheter access for declotting of an upper-extremity hemodialysis loop graft using the crossed catheter technique.
| 1. Obtain antegrade access to the graft near the arterial anastomosis. |