Miguel A. De Gregorio • Alicia Laborda
Massive hemoptysis, also known as life-threatening hemoptysis, is a serious, critical condition that necessitates urgent assessment and treatment of the patient. Despite advances in the treatment of massive hemoptysis, it still imposes a high-risk condition. According to published data, in any given year, 28% of pulmonologists witness a case of death due to massive hemoptysis.1
The lungs receive blood from the pulmonary artery and bronchial systems under normal conditions. In pathologic situations, systemic arteries can penetrate into the lung and irrigate contained injuries. The low-pressure pulmonary system tends to produce small-volume hemoptysis, whereas bleeding from the bronchial system, which shares systemic pressure, tends to be profuse.2
Conservative medical treatment of patients with expectoration of 300 to 600 mL of blood per day is associated with mortality in 50% to 100% of the patients affected,3 with asphyxia rather than hemorrhage commonly being the cause of death.4 Although surgical treatment of massive hemoptysis is associated with mortality rates between 7.1% and 18.2%, it can reach up to 40% when surgery is performed urgently.5
Since embolization of the bronchial artery was first described by Remy et al.6 in 1973, it has become the main option for the treatment of massive hemoptysis, either at first presentation or in case of recurrence. Various studies have demonstrated its effectiveness, safety, and usefulness.7–15 However, surgery plays an important role in the treatment of massive hemoptysis caused by certain diseases, such as pulmonary hydatidosis, bronchial adenoma, and aspergilloma that is refractory to other treatments.16 In such cases, prior urgent bronchial artery embolization facilitates surgical treatment and improves results because it allows scheduled rather than urgent surgery to be undertaken.5 Surgery also represents the treatment of choice when bronchial artery embolization fails repeatedly or is insufficient to control massive life-threatening hemorrhage.17
Currently, when embolization of the bronchial artery fails, the use of endoscopically implanted thrombin preparations has been proposed on the basis of its high success rate.18
CLINICAL BACKGROUND AND PHYSICAL EXAMINATION
Most hemoptysis are generally small, do not require urgent action, and can be treated medically. It is crucial to differentiate hematemesis from hemoptysis. Before assuming a lower respiratory source of the bleeding, it is important to consider whether the blood may be coming from a nonpulmonary source, such as the upper airway or the gastrointestinal tract. Alkaline pH, foaminess, or the presence of pus may sometimes suggest the lungs as the primary source of bleeding rather than the stomach.
Other data such as age, nutritional status, and comorbidities may assist to the diagnosis and management of hemoptysis.
Epistaxis or expectoration without cough suggests that the source of bleeding is in the upper respiratory tract, whereas expectoration accompanied by cough indicates bleeding from the lower bronchial tree.19
It is difficult to quantify the amount of blood expectorated. In general, patients tend to overestimate, and besides, it is difficult to quantify the amount of blood retained in the alveolar space and the bronchial tree.
Clinical symptoms depend on several factors such as the underlying disease and causes of hemoptysis and the importance and amount of bleeding. Fever, hypoxia, tachycardia, and tachypnea are going to be the most important symptoms that may accompany a hemoptysis. The final cause of death in hemoptysis is not hypovolemia due to blood loss but asphyxiation by drowning.
ETIOLOGY
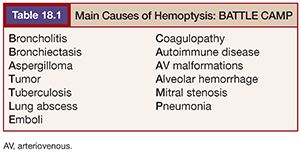
There are many causes of hemoptysis. Their frequency has varied with time. In the past, lung tuberculosis was the first and main cause, but the main cause nowadays is bronchiectasis. Salajka20 has summarized the main hemoptysis causes in the acronym BATTLE CAMP (Table 18.1).
Hemoptysis, which can be life threatening, complicates the course of 50% to 85% of patients with an aspergilloma.21 Tuberculosis can cause massive hemoptysis through multiple mechanisms: active cavitary or noncavitary lung disease can cause small or large amounts of bleeding. Active disease can cause sudden rupture of a Rasmussen aneurysm (aneurysm of the pulmonary artery that slowly expands into an adjacent cavity because of inflammatory erosion of the external vessel wall until it bursts).21
Many inflammatory or immune disorders can produce hemoptysis, such as Goodpasture syndrome, idiopathic pulmonary hemosiderosis, lupus pneumonitis, and Wegener granulomatosis. Other clinical situations can also explain a hemoptysis: coagulopathy, thrombocytopenia, or use of anticoagulants.
Iatrogenic etiology, especially due to either percutaneous or transbronchial lung biopsy, is a cause of hemoptysis, which is usually minor and transient and occurs in 5% to 10% of percutaneous lung biopsies, but massive hemorrhage and death have also been reported. Hemoptysis has been described in 6% of habitual smokers of free-base cocaine (“crack”) and has been associated with diffuse alveolar hemorrhage.
When hemoptysis is recurrent and coincident with menstruation, it can be caused by intrathoracic endometriosis, usually involving the pulmonary parenchyma but occasionally affecting the airways.21
As it has already been commented previously, blood can come from the pulmonary artery. The main causes of pulmonary origin are as follows: pulmonary embolism; pulmonary arteriovenous malformation, either with or without underlying Rendu-Osler-Weber syndrome; and elevated pulmonary capillary pressure (mitral stenosis, significant left ventricular failure, congenital heart disease, severe pulmonary hypertension). Pulmonary artery perforation from a Swan-Ganz catheter can also produce hemoptysis.
Depending on the study, up to 30% of patients with hemoptysis have no cause identified even after careful evaluation. In a series of 67 patients with cryptogenic hemoptysis, prognosis was generally good, and most patients had resolution of bleeding within 6 months of evaluation.22
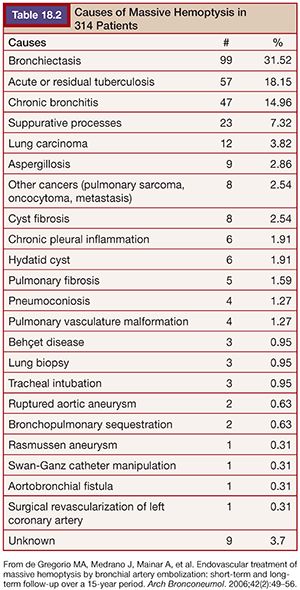
In our series23 of 314 patients, the most frequent causes of massive hemoptysis were bronchiectasis (31.5%), followed by acute tuberculosis or scar tissue caused by tuberculosis (18.1%) and chronic bronchitis (14.9%). Other causes are shown in Table 18.2.
Multislice computed tomography (CT) represents the technique of choice for the etiologic diagnosis of nonmassive hemoptysis. Together with fiber bronchoscopy, they can settle down a very high percentage of the most important and severe causes of hemoptysis. Nevertheless, the use of the CT and the fiber bronchoscopy is discussed in acute massive hemoptysis, where arteriography can establish the diagnosis with good precision and, at the same time, contribute to its treatment.
BRONCHIAL ANATOMY
Bronchial arteries provide oxygen and nutritional elements to different pulmonary structures and also some adjacent mediastinal structures. They supply the trachea, bronchi, visceral pleura, and esophagus and the vasa vasorum of the aorta, pulmonary artery, and vein.24
Although there are many variations, there are usually two bronchial arteries that run in the right lung and one in the left lung. Bronchial arteries originally measure about 1 to 1.5 mm diameter. Above this diameter, they must be considered pathologic.25 The most common source of hemoptysis is located in the bronchial arteries (90%), and only in 5% of cases the pulmonary circulation is the origin of the bleeding.26
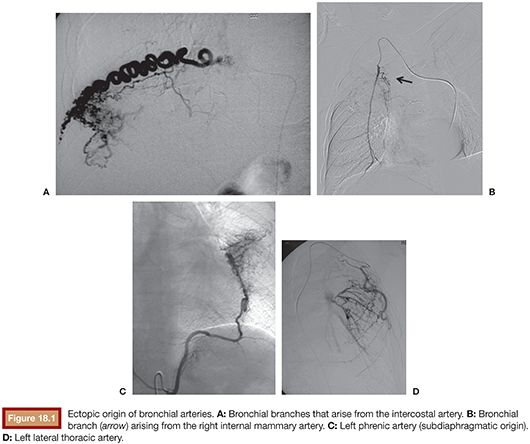
The remaining 5% of pulmonary bleeding originates directly from the aorta or systemic intrathoracic or extrathoracic branches.27–29 In over 70% of the general population, the bronchial arteries arise directly from the descending thoracic aorta, most commonly between the levels of T5 and T6.30 When the bronchial arteries arise at these levels, they are called orthotopic, and when the source is outside these levels, their name is ectopic (8% to 35%). These ectopic bronchial arteries may originate from the aortic arch, brachiocephalic artery, subclavian artery, internal mammary artery, thyrocervical trunk, coronary arteries, costocervical trunk, pericardiacophrenic artery, inferior phrenic artery, or abdominal aorta31 (Fig. 18.1).
According to the classification of Morita et al.,32 the main right bronchial artery arises from the intercostobronchial trunk in more than 50% of the cases, and in the rest, it comes from a common trunk (right and left bronchial arteries) from the subclavian artery or directly from the aorta. Left bronchial artery, on the other hand, arises from a bronchial common trunk (right-left) in over 95% of the cases. Referencing the axial division of the aorta into eight segments, the right intercostobronchial artery ostium is usually located in the medial segment or anteromedial, whereas the origin of the bronchial common trunk (right-left) arises from the anterolateral segment (>70%)33 (Fig. 18.2).
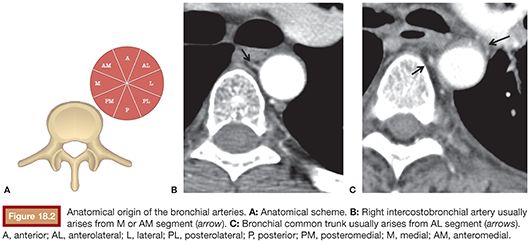
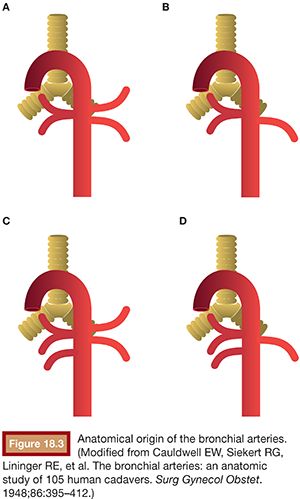
Cauldwell et al.34 reported four classic bronchial artery branching patterns: type I, one intercostobronchial trunk on the right and two bronchial arteries on the left (40%); type II, one intercostobronchial trunk on the right and one on the left (21%); type III, two branches on the right (one intercostobronchial trunk and one bronchial artery) and two bronchial arteries on the left (20%); and type IV, two on the right (one intercostobronchial trunk and one bronchial artery) and one bronchial artery on the left (9.7%) (Fig. 18.3).
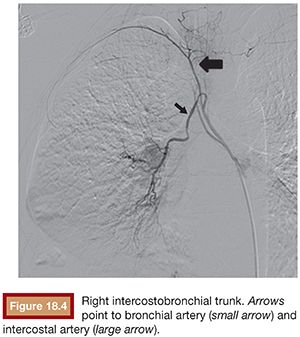
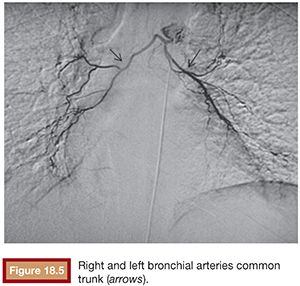
The right intercostobronchial trunk is the most consistently seen vessel at angiography (80% of individuals) (Fig. 18.4). It usually arises from the right posterolateral aspect of the thoracic aorta, whereas the normal right and left bronchial arteries arise from the anterolateral aspect of the aorta. Right and left bronchial arteries that arise from the aorta as a common trunk are not uncommon at angiography (Fig. 18.5). The true prevalence of a common bronchial artery trunk is unknown.
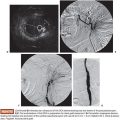
Of particular relevance is the anatomy of the nearby spinal arteries. During bronchial angiography, we can observe two types of spinal arteries that must always be avoided: the dorsal and ventral radicular arteries, which supply the dorsal and ventral nerve root and which emerge from the intercostal arteries, and the anterior medullary arteries. An average of eight arteries supply the spinal cord from its anterior side. In 5% to 10% of cases, they can be observed during bronchial angiography, usually arising from the intercostal artery of the right intercostobronchial trunk. Adamkiewicz artery or great anterior radiculomedullary artery is the most important artery in terms of medullary irrigation. This artery arises from the left side of the aorta, from T9 to L1, in 75% of cases. Anterior medullary arteries have a characteristic “hairpin” configuration at angiography35 (Fig. 18.6).
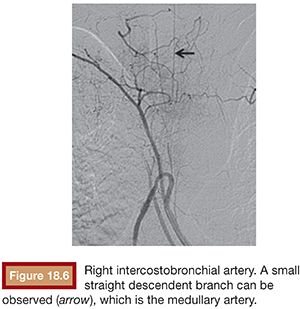
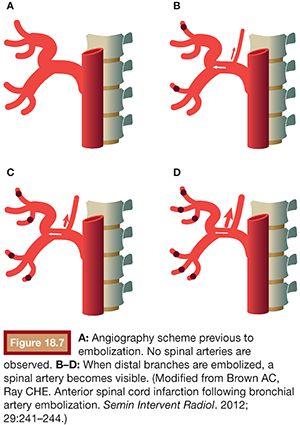
Often, spinal arteries cannot be observed initially in the angiography. They only become visible when the distal vessels are embolized, as recalled by Brown and Ray36 (Fig. 18.7).
WORKUP
Initial priorities are ensuring adequate airway protection, ventilation, and cardiovascular function. Patients with poor gas exchange, rapid ongoing hemoptysis, hemodynamic instability, or severe shortness of breath should be orally intubated with a large-bore endotracheal tube (size 8.0 or greater).15,17 Coagulation disorders should be rapidly reversed.
If the location or site of bleeding is known, placing the bleeding lung in a dependent position may prevent blood spillage into the nonbleeding lung. An alternative strategy involves placement of a typical, single lumen endotracheal tube into either the right or the left mainstem bronchus. The approach of selective intubation is less practical when the right lung is bleeding because selective intubation of the left mainstem bronchus may be difficult. A third alternative is the placement of a double lumen endotracheal tube specially designed for selective intubation of the right or left mainstem bronchi. 21,37
The evaluation should begin with the initial history and physical examination supplemented by chest radiograph. Important features of the history include age, smoking history, duration of hemoptysis, and association with symptoms of acute bronchitis or an acute exacerbation of chronic bronchitis.21
Vital signs including pulse oximetry levels and blood gases, temperature, heart rate, and breathing should be recorded and fever, tachycardia, tachypnea, and hypoxia corrected.
In a survey of respiratory physicians during the 1998 American College of Chest Physicians (ACCP) Annual International Scientific Assembly, more than 50% of these clinicians favored the use of interventional angiography even in surgical patients, which was a substantial change from 1988 when only 23% had favored this approach.1
Location of Bleeding: Computed Tomography versus Bronchoscopy
Diagnostic examination in massive hemoptysis should focus on etiology and identification of the site of bleeding. Such examination commonly involves chest radiography, fiberoptic bronchoscopy, and chest CT.38
Despite the fact that chest radiography is a standard procedure and is always available, it rarely provides clear information on the site of bleeding.
Various studies have reported that fiberoptic bronchoscopy can help to locate the site of the hemorrhage in between 49% and 92.9% of cases.21,23 Fiberoptic bronchoscopy is often considered in patients with hemoptysis and a normal or nonlocalizing chest X-ray to rule out endobronchial malignancy. Performed early in the evaluation, while the patient is actively bleeding, provides the highest performance for locating the bleeding site.1
Many experts advocate the use of fiberoptic bronchoscopy as the primary method of localizing the site of bleeding in massive hemoptysis,39,40 but many studies have downplayed this technique and even set it aside just as an aid to the location of the bleeding before arterial embolization.41
Instead, an early chest CT has been advocated to help localize the bleeding site and diagnose the cause of hemoptysis.42 The advantage of CT is that it may define one of several diagnoses such as bronchiectasis, lung abscess, and mass lesions, including cancer, mycetomas, and arteriovenous malformations. CT can localize the site of bleeding in 63% to 100% of cases. Specifically, CT can aid in diagnosing nonbronchial systemic supply in 80% of cases, with the lowest success in visualizing internal mammary supply.43 Knowledge of nonbronchial supply in advance has the potential for increasing the success rate of initial embolization.21,41 It may also help in acute cases to guide arteriography or bronchoscopy to the regions of highest yield.
Revel and coworkers38 wondered if CT could replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis. The disadvantage of chest CT is that it may require temporary movement of an unstable patient away from intensive care.
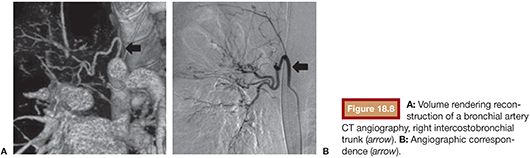
Nowadays with the availability of multislice CT, the use of the fibrobronchoscopy and the arteriography has been questioned for the location and diagnosis of the bleeding. Undoubtedly, the quality of the images, their precision, and the speed and readiness cause CT to play an important role in the diagnosis of the hemoptysis. It has several potential interests, including anatomic workup of bronchial and systemic arterial anatomy to the lung and diagnosis of uncommon sources of bleeding such as pulmonary artery43,44 (Fig. 18.8).
Bronchial and Systemic Angiography
The main objective of a severe hemoptysis diagnosis is the design of an appropriate treatment approach. It is unquestionable that embolization stands out as the optimal treatment. Every embolization requires a previous angiographic study, which is the gold standard for diagnosis of hemoptysis. However, although CT might delay the intervention, it may be very helpful to understand the etiology, the mechanism of bleeding, and the origin of aberrant bronchial or systemic arteries and to plan a successful procedure.45
If the patient continues bleeding and the source is still unknown, then arteriography should be performed next because it may be useful for therapy as well as for diagnosis. Because most massive bleedings arise from the bronchial circulation, bronchial arteriography has a higher performance than arteriography of the pulmonary or systemic arterial beds.
Knowledge of the anatomy of the bronchial arteries is crucial for their location and examination. The bronchial arteries exhibit several anatomic variations in terms of both their origin and their various branches.32,34
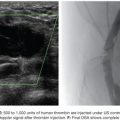
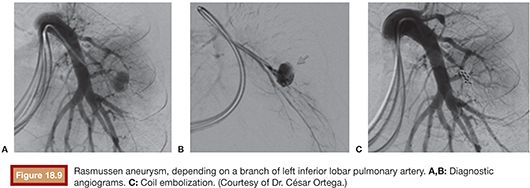
When the pulmonary arterial circulation is the source, the most common underlying conditions are pulmonary arteriovenous malformations, Rasmussen aneurysms, or iatrogenic pulmonary artery tears. A recent study observed that 8 (10.5%) in a series of 76 patients undergoing bronchial angiography for hemoptysis had visible pulmonary artery pseudoaneurysms.46 In our series,23 the pulmonary artery was the origin of pulmonary bleeding in 6 patients (2%) with different causes. In only one case a Rasmussen aneurysm was diagnosed (Fig. 18.9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree