Hepatic Trauma
Hepatic trauma was first graphically described in the Iliad , when Achilles “stabbed with his sword at the liver, the liver was torn from its place, and from it the black blood drenched the fold of his tunic and he was shrouded in darkness as the light went out.” The liver is not faring much better 2600 years later, with the increasing violence of urban life, our society’s passion for high-speed travel, and the gastroenterologist and interventional radiologist constantly invading the liver. The cross-sectional imaging modalities have dramatically improved the promptness and certainty of diagnosis in patients with hepatic trauma and have proved useful in the triage of patients to surgical or conservative management. The single most important factor allowing nonoperative management of blunt hepatic trauma has been the evolution of computed tomography (CT) scanning.
Epidemiology
The types of hepatic injury encountered in practice depend to a large degree on the location of the trauma center. In suburban and rural areas, hepatic injury is usually due to blunt trauma sustained in motor vehicle accidents and falls. In the setting of blunt abdominal trauma, the liver and spleen are the most commonly injured solid organs. The reported prevalence of liver injury in patients who have sustained blunt trauma ranges from 1% to 8%. Other associated injuries are present in 80% of people. In large city hospitals, penetrating injuries from firearms and stabbings predominate. In this setting, the liver is the most frequently injured viscus owing to its anterior and partially subcostal location. Iatrogenic hepatic injuries are becoming increasingly common in all hospitals. Indeed, liver biopsy is the most common cause of subcapsular hematoma in the United States today. Because of their markedly different management paradigms and prognoses, blunt, iatrogenic, and penetrating traumatic injuries are discussed separately in this chapter.
Clinical Findings
Clinical features associated with hepatic trauma include right upper quadrant pain and tenderness with guarding and rebound tenderness, falling hematocrit, and hypotension. Delayed manifestations of biliary trauma, such as bilomas, are usually accompanied by right upper quadrant pain and jaundice. Trauma is the most frequent cause of hemobilia, which is manifested by hematemesis or melena associated with right upper quadrant pain. Because of the difficulty of identifying hepatic injury by physical examination, CT is often required to avoid missing a significant injury. The mortality rate reported secondary to blunt liver trauma ranges from 4.1% to 11.7%. The American College of Surgeons state a mortality rate of 16.8%, taking all hepatic injuries into account on the basis of the National Trauma Data Bank.
Diagnostic peritoneal lavage was one of the most common clinical modalities used in the diagnostic evaluation for blunt abdominal trauma in the mid-20th century. This procedure is sensitive for hemoperitoneum but led to a high rate (30%) of nontherapeutic, unnecessary laparotomies. Diagnostic peritoneal lavage has largely been replaced with CT or screening ultrasound for the evaluation of hemoperitoneum.
Classification of Hepatic Trauma
The management of hepatic trauma patients has evolved significantly during the last three decades and is now based on well-defined treatment algorithms. This has highlighted the need for an accurate classification system as a basis for the clinical decision-making process. Fundamental to the development of these classification schemes and the subsequent treatment algorithms based on them is the widespread availability of rapid acquisition CT imaging in most major trauma centers. This has also led to a diminution in the role of diagnostic peritoneal lavage in the management of the trauma patient. Diagnostic peritoneal lavage has multiple limitations: lack of specificity regarding the source of bleeding; high sensitivity to detection of small quantities of blood, leading to nontherapeutic laparotomy; and inaccuracy for retroperitoneal injuries. Diagnostic peritoneal lavage fails most importantly in its ability to identify the location and extent of intra-abdominal organ injury. Diagnostic peritoneal lavage is valuable mainly in patients in whom ultrasonography or CT scanning is inappropriate or unavailable.
Previous attempts to categorize hepatic injury were mostly anatomic in design and did not incorporate the mechanism of injury or physiologic changes associated with more severe injuries. In 1987, the Organ Injury Scaling Committee of the American Association for the Surgery of Trauma was organized for the purpose of devising injury severity scales for individual organs. These injury severity scales incorporated etiology, anatomy, and extent of injury and correlated it with subsequent clinical management and outcome. The most recently revised clinical classification is listed in Table 91-1 . It has been demonstrated that the CT grade of hepatic injury is not predictive of the need for surgery and that the majority of injuries can be safely managed nonoperatively. The hemodynamic status of the patient may be the most important predictor of injury severity; however, patients with higher grade injuries (IV or V) are more likely to be unstable and to require surgery, and increasing organ injury severity scale grade in patients with isolated hepatic injuries is associated with increasing mortality. In contrast, patients who are stable may be managed conservatively with close observation in intensive care units. Mechanism of injury, radiologic classification, and clinical assessment of hemodynamic stability must all be correlated by the trauma surgeon to guide management decisions.
| Grade | Injury | |
|---|---|---|
| I | Hematoma | Subcapsular, <10% surface area |
| Laceration | Capsular tear, <1 cm parenchymal depth | |
| II | Hematoma | Subcapsular, 10%-50% surface area Intraparenchymal, <10 cm diameter |
| Laceration | 1-3 cm parenchymal depth, <10 cm in length | |
| III | Hematoma | Subcapsular, >50% surface area or expanding; ruptured subcapsular or parenchymal hematoma Intraparenchymal hematoma >10 cm or expanding |
| Laceration | Parenchymal fracture >3 cm deep | |
| IV | Laceration | Parenchymal disruption involving 25%-75% of hepatic lobe or 1-3 Couinaud segments within a single lobe |
| V | Laceration | Parenchymal disruption involving >75% of hepatic lobe or >3 Couinaud segments within a single lobe |
| Vascular | Juxtahepatic venous injuries (i.e., retrohepatic vena cava/central major hepatic veins) | |
| VI | Vascular | Hepatic avulsion |
Pathophysiology
Blunt Trauma
Approximately 1% to 8% of patients who sustain blunt abdominal trauma have a liver injury. Severe compressive trauma to the liver from a steering wheel injury or direct blow can produce satellite fractures that often involve an entire lobe. Rapid deceleration in motor vehicle accidents produces shearing forces that cause different degrees of parenchymal tears. The hepatic lobes may be torn from each other, or the tears may involve the supporting ligaments, hepatic veins, and inferior vena cava. The most frequent site of hepatic injury is the posterior segment of the right lobe of the liver because of its size and proximity to the ribs and spine. Although they are less common, left lobe injuries are more often associated with retroperitoneal injuries (duodenum and pancreas) and transverse colon injuries. There is a high incidence of associated extrahepatic injury, including splenic rupture, head injuries, rib fractures, facial fractures, and pelvic fractures.
Major hepatic venous injuries occur in 13% of patients with liver trauma. These are most often a result of blunt trauma. They include, in decreasing order of frequency, right hepatic vein avulsion from the inferior vena cava, upper branch right hepatic vein avulsion, avulsion of accessory veins, avulsion of the left hepatic vein, and avulsion of the middle hepatic vein. The right hepatic vein is at greater risk for sudden acceleration-deceleration injury because it has a relatively long extrahepatic segment before it enters the inferior vena cava. The course is shorter for the middle and left hepatic veins, which usually merge to form a common channel and thus are less frequently injured. Venous injuries can be suggested by multiple lacerations around the inferior vena cava or porta hepatis. It is important to identify these findings because they may help prepare the surgeons to expect significant bleeding when the liver is lifted off the inferior vena cava. Multiplanar CT reconstruction and three-dimensional rendering can be helpful in the assessment of vascular involvement, especially the portal vein, hepatic veins, and retrohepatic vena cava. Injuries of the bare area of the liver may not demonstrate peritoneal signs or findings on diagnostic peritoneal lavage or screening sonography for hemoperitoneum but may show retroperitoneal blood on CT examination.
Penetrating Injuries
Penetrating wounds are most commonly caused by stabbing, gunshot, or shotgun injury. Knife stabbings usually cause superficial lacerations, whereas high-velocity projectiles generally cause injury from capsule to capsule, with massive parenchymal damage.
Iatrogenic Injuries
The liver is subject to a number of iatrogenic misadventures that may be caused by any of the various needles, wires, cannulas, and catheters placed by gastroenterologists and interventional radiologists. The liver can also be damaged by external cardiac compression during the course of resuscitation and by inappropriately low insertion of a chest tube.
Diagnostic and interventional procedures can produce a tear of the liver capsule, subcapsular hematoma, bile leak, arteriovenous fistula, pseudoaneurysm, arteriobiliary or venobiliary fistula, hepatic hematoma, hemoperitoneum, and biloma. The prevalence of this problem was well documented in one study in which intrahepatic (77%) and subcapsular (23%) hematomas were found sonographically in 23% of asymptomatic patients evaluated after liver biopsy.
Spontaneous Rupture
Hepatic rupture and hemorrhage can occur in eclamptic or preeclamptic women during the third trimester due to h emolysis, e levated l iver enzymes, and l ow p latelets, the HELLP syndrome. The maternal mortality rate approaches 60% with hepatic rupture. Because surgery is difficult in these friable livers, hepatic artery transcatheter embolization offers a therapeutic alternative. One report showed good results with absorbable gelatin foam (Gelfoam) embolization in four eclamptic patients. Bleeding from the liver may also immediately follow delivery in noneclamptic women.
Spontaneous hemorrhage can occur in patients who have sickle cell anemia, peliosis, hepatomas, hepatic adenoma, coagulopathies, B-cell lymphoma, metastases, organophosphate toxicity, or collagen-vascular disease and in patients who receive long-term hemodialysis.
Radiologic Findings
Imaging can make a major contribution to the management of trauma patients who are hemodynamically stable and to the postoperative assessment of these patients. *
* References .
Computed Tomography of Hepatic Injuries
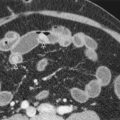
Multidetector computed tomography (MDCT) is the imaging technique of choice for hepatic trauma and has had an enormous impact on the detection and management of liver injuries. † It can reliably diagnose and stage significant hepatic and extrahepatic injuries, document interval healing of hepatic injuries, and diagnose early and delayed complications. It can also detect associated and unsuspected injuries to other organs ( Fig. 91-1 ). Patients who are hemodynamically unstable require immediate surgery; surgical delay secondary to imaging can be fatal. Hemodynamically stable patients can be scanned in a matter of seconds, with better resolution and with multiplanar imaging capabilities, given our current CT technology.

† References .
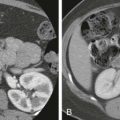
With any type of abdominal trauma, the first finding that should be evaluated is the presence or absence of blood, either parenchymal or intraperitoneal (hemoperitoneum). Immediately after injury, hematomas on noncontrast scans are hyperdense relative to normal hepatic parenchyma ( Fig. 91-2 ). After intravenous administration of contrast medium, unclotted blood is usually hypodense compared with enhancing normal parenchyma but still usually has an attenuation (20-40 HU) greater than that of simple fluid (0-20 HU).
Active hemorrhage is identified as extravasation of contrast material on contrast-enhanced CT. The attenuation values of extravasated contrast material (155 HU, mean value) and hematoma (54 HU, mean value) help distinguish active bleeding from clotted blood. If the liver is involved by focal or diffuse fatty infiltration, a common finding in intoxicated drivers, parenchymal hematomas may appear isodense on contrast-enhanced examinations but can usually be distinguished with narrow CT window settings. In addition, the attenuation and the distribution of fluid can serve as a clue to the site of injury. This phenomenon has been termed the sentinel clot sign. The sentinel clot is the highest density blood collection seen and usually lies adjacent to the injured organ ( Fig. 91-3 ). The evaluation of hepatic trauma should also search for other fluid collections, such as bile, as seen in bile leaks and bilomas, which have low attenuation because of their high cholesterol content.

In interpreting CT scans in patients with hepatic trauma, it is important to describe the anatomic site and extent of injury (superficial, deep, lobar, segmental, perihilar), the complexity of the lesion (simple or stellate laceration), and the type of hepatic injury (parenchymal laceration or subcapsular hematoma).
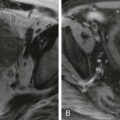
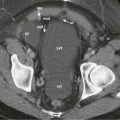
The major CT findings in blunt hepatic injuries are lacerations, subcapsular and parenchymal hematomas, active hemorrhage, and juxtahepatic venous injuries. Lacerations are the most common injury and appear as branching or linear low-attenuation areas. Lacerations can be classified into superficial (<3 cm from the liver surface) and deep (>3 cm from the surface) ( Fig. 91-4 ). Deep central parenchymal injuries seen on CT may be unrecognized at laparotomy, especially if the liver surface appears intact, and as a result, the true extent of involvement may be underestimated at surgery. It is imperative to describe any potential laceration extension to the hepatic or portal veins or to the inferior vena cava ( Fig. 91-5 ; see also Fig. 91-3 ). Hematomas can also occur in hepatic trauma and may be subcapsular or parenchymal in nature. Subcapsular hematomas can be distinguished from free hemoperitoneum by mass effect on the liver surface, creating a contour deformity. Parenchymal hematomas follow the same attenuation values of blood as described before. Again, on contrast-enhanced CT, they may appear relatively lower in attenuation than enhancing liver parenchyma, but their attenuation values are often higher than simple fluid ( Fig. 91-6 ). Yet another type of hepatic injury, contusion, often appears as low-attenuation, sometimes ill-defined areas with intermixed areas of high attenuation representing blood.



Active extravasation on contrast-enhanced CT represents one of the more severe CT features of hepatic injury and can herald life-threatening, active bleeding ( Figs. 91-7 and 91-8 ). On CT, this appears as very high attenuation approximating that of aorta (91-274 HU; mean, 155 HU) compared with the density of clotted blood (28-82 HU; mean, 54 HU). At times, active extravasation of contrast material may be difficult to distinguish from a pseudoaneurysm. Delayed contrast images are helpful in this instance, demonstrating “washout of contrast attenuation” with pseudoaneurysms (approximating the blood pool density at that time) and persistence of high density with active bleeding. Active extravasation of contrast material has been shown to be a strong predictor of potential cardiovascular collapse and failure of nonsurgical management. Treatment of active extravasation is best performed through angiographic embolization.


Vascular injuries of the liver, although relatively rare, can be fatal as well. When these occur, they are usually due to lacerations extending to the inferior vena cava, hepatic veins, and portal veins; thus, it is imperative to identify and to relay this extension to the clinicians (see Fig. 91-3 ). Alternatively, avulsion of the vessels can occur as well. Inferior vena cava injuries carry a particularly high mortality rate and should be suspected when there is a laceration extending to the proximal hepatic veins and inferior vena cava and when there is a large amount of retrohepatic blood. If bleeding occurs at the bare area of the liver, there may be extension of blood into the retroperitoneum as well.
Periportal zones of decreased density may be the only manifestation of hepatic trauma. These may represent linear collections of blood in the periportal regions or dilated periportal lymphatics. Periportal low density was previously thought to be an important sign of liver injury but is actually most commonly due to rapid fluid resuscitation, elevating central venous pressure, as suggested by a distended inferior vena cava ( Fig. 91-9 ). Periportal low density may also be seen in nontraumatic causes, including in patients with congestive heart failure, patients with hepatitis, liver transplant recipients, and those with AIDS. The lucency is due to a dilation of the intrahepatic lymphatics caused by obstruction of drainage. Intraparenchymal gas, in the absence of infection, has also been reported in patients with blunt abdominal trauma, but an abscess must always be excluded when extraluminal gas collections are present.

A CT-based classification has been formulated from these findings ( Table 91-2 ). This CT classification system grades the injuries; however, this grading system and the CT features may not correlate with the need for surgery. It is not the grade of the injury but rather the hemodynamic parameters of the patient that often dictate conservative versus operative management decisions. Even major hepatic injuries up to and including grade IV can usually be managed conservatively if the patient is hemodynamically stable. CT findings associated with increased morbidity, mortality, and need for operation include deep perihilar lacerations, failure of the hemoperitoneum to be significantly resorbed within 1 week, rapid progression of hepatic injuries within hours or days of injury, and major vascular trauma (especially injury to the confluence of hepatic veins). It has been noted that many hemodynamically stable patients can avoid operation despite significant injuries seen on CT examination. Although the CT-based injury grading system may not correlate with the need for surgery, it serves as a consistent means of describing and communicating injuries to surgeons. Radiologists should therefore be familiar with this grading system.
| Grade | Criteria |
|---|---|
| 1 | Capsular avulsion, superficial laceration(s) <1 cm deep, subcapsular hematoma <1 cm maximal thickness, periportal blood tracking only |
| 2 | Laceration(s) 1-3 cm deep, central/subcapsular hematoma(s) 1-3 cm diameter |
| 3 | Laceration(s) >3 cm deep, central/subcapsular hematoma(s) >3 cm diameter |
| 4 | Massive central/subcapsular hematoma >10 cm, lobar tissue destruction (maceration) or devascularization |
| 5 | Bilobar tissue destruction (maceration) or devascularization |
The need for follow-up CT examination to detect delayed complications is favored by some and opposed by others. Patients with persistent or worsening symptoms should have follow-up examinations. Patients with more severe, complex hepatic injuries may also benefit from repeated CT to evaluate injury status and possible complications. Meredith and colleagues found that hemodynamic stability and lack of peritoneal findings were more predictive than the CT findings of which patients could undergo nonoperative management.
Post-traumatic complications are not uncommon after hepatic injury and are best demonstrated by CT as well. Delayed hemorrhage can occur and can have a high mortality rate. This may be due to several causes, including an expanding injury, a biloma-induced pseudoaneurysm, a misinterpretation of contrast material extravasation on the initial CT scan, and mismanagement of the patient’s hemodynamic status ( Fig. 91-10 ). This should be suspected if there is a delayed drop in the hematocrit level. Post-traumatic pseudoaneurysms of the hepatic artery and its branches may occur weeks to months after the initial injury. These patients present with delayed hemorrhage and hemobilia (if the pseudoaneurysm decompresses into the biliary system) and should be evaluated with hepatic angiography, at which time the aneurysm can be embolized to minimize the risk of frank rupture. Bilomas ( Fig. 91-11 ) and intrahepatic and perihepatic abscesses are other major post-traumatic complications. Bilomas are seen in 0.5% to 20% of patients managed nonoperatively. These fluid collections usually resolve or can be managed successfully by percutaneous drainage. Aspiration, biliary scintigraphy, or magnetic resonance imaging (MRI) may be necessary for differentiation of these fluid collections. Endoscopic retrograde cholangiopancreatography (ERCP) can identify the bile leak and guide the insertion of a biliary endoprosthesis.


Ultrasound
Ultrasound is being more frequently performed in the emergency department by surgeons, radiologists, and emergency department physicians. The advantages of sonography include portability, ability to rapidly detect intraperitoneal blood, and relative inexpense. Problems include the operator-dependent nature of ultrasound and limitations in demonstrating the extent of injury.
The use of ultrasound was pioneered years ago in the European literature. Ultrasound can be used either to identify parenchymal injury directly or to detect intraperitoneal fluid that in the acute setting is presumed to represent hemoperitoneum. Ultrasound is much more accurate in the detection of hemoperitoneum than in the diagnosis of specific parenchymal or hollow viscus injury. The recent interest in ultrasound is primarily in its utility as a rapid screening test for significant injury, the so-called focused abdominal sonogram for trauma (FAST). FAST simply entails sonographic interrogation of sites in which free intraperitoneal fluid most often accumulates: Morison’s pouch, the left subphrenic and subsplenic areas, and the pouch of Douglas. It also involves evaluation for the presence of hemopericardium. According to Ma and coworkers, the suprapubic view is the single view most sensitive for hemoperitoneum (68%), but the sensitivity dramatically increases when multiple views are obtained. The best use of FAST is to triage patients. A hemodynamically stable patient with an abnormal finding on FAST should undergo CT scanning, whereas an unstable patient with an abnormal ultrasound finding should go to surgery emergently. Clinical management in cases with a normal finding on FAST is more nebulous, but at a minimum, those patients should at least be observed for a time.
Multiple studies have been published looking at the accuracy of ultrasound and comparing it with other modalities. In general, ultrasound is relatively sensitive for the detection of free intraperitoneal fluid, but it is far from perfect. There are a wide range of values in the literature for sensitivity (81%-94%), specificity (88%-100%), and accuracy (86%-98%) for free intraperitoneal fluid. This type of variability suggests inconsistent reproducibility of the method. In addition, for these values to be attained, some studies required repetitive sonographic scanning to detect developing fluid. Another confounding factor is that not all blunt abdominal trauma causes free fluid, with false-negative results. In some studies, 26% to 34% of patients with abdominal organ injuries did not have hemoperitoneum. Therefore, although ultrasound is very good in detecting hemoperitoneum, there are false-negative and false-positive findings. Diagnostic peritoneal lavage is a method to detect hemoperitoneum that was used commonly before the advent of ultrasound and CT. Diagnostic peritoneal lavage is known to be more sensitive than ultrasound in the detection of fluid and injury; however, positive diagnostic peritoneal lavage results led to a 30% rate of nontherapeutic, unnecessary laparotomies. Diagnostic peritoneal lavage has been largely replaced with ultrasound and CT. In addition to the evaluation for hemoperitoneum, ultrasound can be used to detect other injuries. Ultrasound can demonstrate a number of traumatic lesions: subcapsular hematomas, parenchymal tears, contusions, and bilomas. A subcapsular hematoma appears as a lentiform or curvilinear fluid collection with echogenic properties that vary with the age of the lesion ( Fig. 91-12 ). Hematomas are initially anechoic, but as clotting proceeds, they become progressively echogenic by 24 hours. As time passes, the hematoma’s echogenicity begins to decrease again. Internal echoes and septations develop within these collections in 1 to 4 weeks. The frequency of the transducer also determines the sonographic characteristics of the hematoma. The complexity and echogenicity of the hematoma can appear greater at higher frequencies as a result of higher spatial resolution.

Parenchymal contusions are normally hypoechoic at initial presentation, transiently become hyperechoic, and then become hypoechoic. Lesions that occur posteriorly in the right lobe or over the spine within the left lobe are usually not sonographically demonstrable unless the patient has a slender body habitus. Parenchymal tears, with or without hematoma, are manifested as irregular defects with abnormal echotexture relative to the surrounding normal tissue. Intraparenchymal hematomas as small as 3.5 mL can be visualized as rounded, echogenic foci.
Bilomas appear as rounded or ellipsoid, anechoic, loculated structures with sharply defined margins close to the liver and bile ducts. Scintigraphy is an important adjunct in these cases to show communication of the lesions with the normal biliary tract. Fluid aspiration may be helpful to demonstrate bile. Ultrasound is particularly helpful diagnostically when the collections no longer communicate with the biliary tract. Percutaneous drainage with CT or sonographic guidance may be helpful when fluid collections are symptomatic or infected.
Many studies have evaluated the accuracy of ultrasound for detection of parenchymal hepatic and other solid organ injuries. Sensitivity for detection of all injuries ranges from 43.6% to 93%. This wide range of numbers is due to many factors, including study design, technique, and experience. Papers with very low sensitivities often include small bowel and mesenteric injuries, which are extremely difficult to detect by ultrasound. With respect to liver parenchymal injury specifically, an article by Sato and Yoshii comparing ultrasound examination with both the clinical outcome and CT revealed a sensitivity for hepatic injury of 87.5% among experts and 46.2% among nonexpert sonographers. This suggests that detection of injuries sonographically is more difficult than simply scanning for hemoperitoneum and can be variable. Other pitfalls of ultrasound, however, include limitations in the evaluation of retroperitoneal injuries, diaphragmatic injuries, and hollow viscus perforations.
Some authors have experimented with contrast-enhanced ultrasound in the evaluation of hepatic trauma. These contrast agents use stabilized, encapsulated microbubbles that are small enough to pass through the pulmonary circulation to reach the systemic circulation and ultimately parenchymal tissue. Contrast agents have many potential uses in the heart and vascular system as well as in the detection of focal lesions. In the trauma setting, Catalano and associates have demonstrated that contrast-enhanced sonography is a promising tool that better depicts hepatic lacerations and hematomas as echo-poor areas against a background of enhanced parenchyma. It also appears to demonstrate the extent of lesions better than conventional sonography. In addition, it may also have a role in detecting active extravasation of contrast material and therefore active bleeding, which cannot be done with conventional ultrasound. In their study, contrast-enhanced sonography had a higher sensitivity for hepatic injury detection compared with routine ultrasound, 87% versus 65%. This may potentially be a powerful tool in the emergency department setting in the future.
Nuclear Scintigraphy
Bile leaks are rare in blunt hepatic trauma. They more often follow cholecystectomy and partial hepatectomy. Hepatobiliary scans, the most sensitive means of detecting bile leakage, may identify the leak before the onset of clinical symptoms. With bile leakage, the tracer may appear as a subcapsular collection or may pool freely in the peritoneal cavity. Parenchymal injuries such as lacerations and hematomas are easily identified on early hepatic phase images.
Magnetic Resonance Imaging
Gadolinium-enhanced MRI can depict complex hepatic injuries in patients with contraindications to iodinated contrast material. Contrast-enhanced MRI has been shown to demonstrate traumatic hepatic injuries equal to and, occasionally, better than contrast-enhanced CT. MRI, however, requires a longer imaging time as well as the need to accommodate metallic life support and monitoring equipment, which is more difficult than with CT. In addition, the MR screening process, which is necessary for the safety of the patient, is a timely process that is often not possible in a trauma setting where immediate decisions need to be made. Furthermore, images are compromised by motion to a greater extent than with CT as well as by limited evaluation of the bowel. MRI can help differentiate bilomas from subacute hematomas. Despite these limitations, MRI may have a role in imaging of the trauma patient, although its use has not been thoroughly evaluated. With the advent of gadolinium-based hepatobiliary agents such as gadobenate dimeglumine (MulitHance; Bracco Diagnostics) and gadoxetate (Eovist; Bayer HealthCare), the biliary tree can be evaluated in a functional way. These hepatobiliary agents have dual excretion through the kidneys and liver. The contrast agent is taken up by the hepatocytes and excreted through the biliary tree. Approximately 50% of gadoxetate is excreted through the liver, whereas 5% of gadobenate has hepatobiliary excretion. This biliary excretion allows visualization of the bile ducts and any bile leaks or bilomas on T1-weighted MR sequences, on which the gadolinium portion of these agents will cause T1-weighted shortening and increased signal on T1-weighted images. Imaging for the hepatobiliary phase of biliary excretion is done in a delayed fashion after routine images. With Eovist, a delay of approximately 20 to 30 minutes is used; with MultiHance, a delay of 60 minutes is optimal. The few studies performed for the evaluation of bile duct injury have been in iatrogenic injuries after surgery. In this setting, MR has been shown to find and to characterize bilomas with a sensitivity of 80% to 96%. Many other types of biliary abnormalities, including strictures, leaks, obstructions, and anatomic anomalies, can be depicted with these agents ( Fig. 91-13 ). Although it is still a Food and Drug Administration off-label application of these agents, functional biliary imaging with these contrast agents is extremely promising. Apart from identifying biliary injury, MRI may also have a role in follow-up of previously detected hepatic injuries. In severe hepatic injury in which follow-up imaging is warranted, the use of MRI over CT may save a significant radiation dose to often young patients, particularly if multiple follow-up examinations may be needed.

Angiography
Once the mainstay of hepatic trauma diagnosis, angiography is now usually performed to investigate and to treat severe and active hemorrhage, post-traumatic fistulas, and pseudoaneurysms. Angiography, however, plays a pivotal role in the conservative management of hepatic injury. One of angiography’s main roles is to treat active bleeding, which appears as extravasation of contrast material on CT. In this capacity, angioembolization has a reported efficacy of 83% in controlling bleeding after blunt hepatic injury according to Carrillo and associates. Numerous other studies have shown even greater success of angioembolization in the control of bleeding, minimizing transfusions and need for surgery. Traumatic pseudoaneurysms, arteriobiliary fistulas, arteriovenous fistulas, and portobiliary fistulas are the major sources of occult or delayed hemorrhage in the traumatized liver. These vascular abnormalities are amenable to transarterial embolization with either Gianturco coils or detachable balloons. Gelfoam and Ivalon usually produce only transient hemostasis in patients who have had multiple blood transfusions. Transcatheter arterial embolization has been demonstrated to be an effective alternative to surgery in patients with high-grade (Mirvis classification 3 or 4) injuries to the liver and, in one series, was successful in all patients. Interventional radiologists can manage some of the most lethal venous injuries, including lacerations of the inferior vena cava, with stenting.
Plain Radiographs
Plain radiographic findings are neither sensitive nor specific in patients with hepatic trauma. Nearly 50% of patients with hepatic trauma have fractures of the right lower ribs. When hemoperitoneum is present, there may be loss of the inferior liver-fat interface. Hepatomegaly, irregularity of the liver margin, and caudal displacement of the hepatic flexure all suggest liver injury. Pulmonary contusion, pneumothorax, elevation of the right hemidiaphragm, pleural effusion, hemothorax, and diaphragmatic irregularity frequently accompany hepatic trauma as well.
Treatment
Management of blunt abdominal trauma has undergone a complete paradigm shift in the past two decades. Whereas 80% to 90% of injuries were treated operatively in the 1990s, the majority of patients with blunt hepatic trauma and penetrating liver injuries are now managed nonoperatively if they are hemodynamically stable. As previously stated, it is not the grade of the liver injury but rather the hemodynamic parameters of the patient that determine operative versus conservative management. Validation of conservative management has been substantiated by lower acute mortality rates and acceptable short-term morbidity and complications relative to exploratory surgery and emergent hepatic resections. In patients with American Association for the Surgery of Trauma grade I to grade III liver injuries, the need for surgical exploration after initial resuscitation and observation is usually less than 10% to 20%. If patients with grade IV to grade VI injuries can be nonoperatively stabilized, a period of resuscitation and CT imaging may improve the ultimate outcome of emergent surgery by preoperatively defining the extent and location of specific hepatic injuries. Any nonoperative management, however, requires capabilities for close monitoring and operating room availability for potential urgent intervention. Also, adjunctive therapies including embolization by interventional radiology, percutaneous drainage, endoscopy, ERCP, and laparoscopy need to be readily available.
In patients with severe trauma in whom there is massive bleeding, large amounts of devitalized tissue, or massive bile leak and in the clinical scenario of rebleeding, persistent decline of hemoglobin, or failure of embolization, operative intervention is considered. First, treatment of shock and of head and thoracic injuries has priority; next, abdominal injuries should be addressed. For severe liver injury (grade IV or V), three main surgical principles are control of hemorrhage, drainage of infection, and repair of the biliary system. Bleeding is controlled by specific ligation or direct control of bleeding points; débridement and ligation; hepatic artery ligation; more extensive hepatic resection; tamponade with perihepatic packing; and in extreme cases of venous avulsion, vena cava occlusion, with or without shunting, or cava resection with graft replacement. Injuries to the hepatic veins are the major cause of immediate death from hepatic trauma and are difficult to repair. In patients who decompensate during surgery, damage control surgery can be done; this consists of perihepatic packing and partial closure of the abdominal incision to allow the patient to go to the intensive care unit for resuscitation and correction of any metabolic derangements and then to return to the operating room when the condition is more stable. In patients with extreme uncontrolled bleeding, liver transplantation has been performed in rare cases. Patients with a cerebral intraparenchymal bleed demonstrated on head CT scans are difficult to manage because they may be harmed by the effects of general anesthesia and the fluid shifts that accompany laparotomy, evacuation of hemoperitoneum, and repair of the liver injury. Operative hemostasis and adequate débridement may be difficult, and hepatic surgery carries the risk of postoperative sepsis. After operative control of hemorrhage, the liver is drained externally. Finally, biliary injury must be recognized and repaired as it will not heal spontaneously. In patients with penetrating injury, nonoperative management is attempted for stab wounds and low-velocity gunshot wounds in stable patients in the absence of other injuries. If the patient is unstable or has associated bowel injury, surgery is then mandated.
Complications
Although the need for surgery is not dependent on the CT grade of injury, the grade of injury and CT findings can prognosticate the chance for the development of complications. Patients with higher grade injuries (grades III-V) are more likely to experience complications. According to Kozar and coworkers, 21% of patients with a grade IV injury and 63% of patients with a grade V injury developed hepatic complications. Therefore, with high-grade injuries that undergo nonoperative management, complications should be anticipated, and access to means of treating and monitoring these complications should be readily available.
Bleeding
Bleeding is the most common complication that occurs after trauma. The majority of the time, this can be managed successfully with angioembolization, but rarely surgery is required. Most episodes of bleeding occur early, either on the day of the injury or shortly thereafter. There can be cases of delayed hemorrhage that are due to either delayed rupture of a hematoma or pseudoaneurysm formation. Angioembolization is a vital tool in the management of bleeding at any time during treatment.
Infection
Sepsis and hepatic abscess are serious complications that are far more common with operative than with nonoperative management. Hematomas and bilomas are excellent media for the development of infection. In traumatized patients, bacteria may enter the body through intravenous sites, various indwelling catheters, surgical drains, and other injured areas and by translocation from the gastrointestinal tract. In patients with a suspected intra-abdominal source of sepsis, all intrahepatic or perihepatic fluid collections seen on imaging studies should be viewed with suspicion as a source of infection.
Biliary Tract Complications
Intrahepatic or extrahepatic biliary tract complications, including biliary leaks, biloma, and bile peritonitis, are being detected with increasing frequency in patients with hepatic trauma because of the increased sensitivity of cross-sectional imaging and hepatobiliary scintigraphy. These lesions may take days to weeks to become manifested clinically owing to the slow rate of leakage from the injured bile duct. Most bilomas are asymptomatic and detected by imaging. CT scans and sonography demonstrate nonspecific fluid collections. Most often, bilomas and bile duct injury can be adequately treated by percutaneous drainage or ERCP stenting, but in rare cases, surgery may be needed.
Hepatic Surgery
Hepatic surgery has been likened to an exercise in hemostasis. No bloodless planes exist, as the liver’s complex inflow and outflow tracts course at right angles. The liver parenchyma is also friable and soft and has few landmarks. These factors had hindered the evolution of hepatic surgery to such a degree that before the 1950s, segmental hepatectomy was reserved almost exclusively for trauma. With advances in surgical techniques and anesthesia and a better understanding of internal vascular anatomy ( Fig. 91-14 ) and hepatic physiology, partial hepatectomy has become a safe and accepted mode of therapy for selected patients with primary and metastatic liver tumors as well as certain benign disorders ( Table 91-3 ).

| Indication | Frequency (%) |
|---|---|
| Metastatic tumor | 60 |
| Primary malignant neoplasm | 15 |
| Undiagnosed mass | 8 |
| Benign tumor or cyst | 7 |
| Trauma | 5 |
| Localized biliary abnormality | 3 |
| Infection | 2 |
Two major principles are fundamental to planning of hepatic resection. First, there must be a sufficient amount of hepatic parenchyma to sustain life after surgery. The liver possesses a remarkable capacity to regenerate itself; up to 80% can be safely removed in many patients. Regeneration of liver volume is an efficient, progressive process that in animal studies requires approximately 4 months after an 80% to 85% resection. Coexisting hepatocellular diseases such as cirrhosis decrease the amount of hepatic reserve so much that some patients are unable to tolerate removal of any tissue. Indeed, total hepatectomy and liver transplantation may be the best way to treat patients with small hepatomas or severe cirrhosis.
The second major principle of hepatic resection is preservation of blood supply to the liver tissue left in situ. Adherence to this principle is difficult because the vascular supply is not apparent to the surgeon on inspection of the hepatic surface. In addition, hepatic veins and arteries do not lie in parallel courses, as they generally do in other organs.
Radiology in Surgical Planning
Imaging studies play an important role in identifying the extent of disease that is important for prognosis and therapy. For example, the number and size of hepatic metastases from colorectal neoplasms and extrahepatic involvement determine long-term survival in patients with hepatic resection. The radiologist can help the surgeon adhere to these principles by providing the following information before hepatic resection :
Anatomic location of the lesion in relationship to the portal and hepatic veins, fissures, coronary and falciform ligaments, and fissure for the ligamentum venosum
Presence of vascular invasion of the hepatic vessels by a neoplasm
Bland or tumor thrombus in portal and hepatic veins distant from the lesion of interest
Involvement and patency of the inferior vena cava
Spread of tumor into adjacent structures (e.g., diaphragm, colon, duodenum, lymph nodes)
Confirmation to the maximal extent possible that the lobe of liver to remain in situ is free of tumor
Preoperative studies should include a chest radiograph and chest CT scan to exclude extrahepatic metastases. MDCT or MRI of the liver and the remainder of the abdomen should also be obtained. Removal of multiple liver metastases is possible in some patients.
A common anomaly, the replaced right hepatic artery arising from the superior mesenteric artery, is important for the surgeon to appreciate preoperatively. Other vascular and biliary variations should be noted as well. The placement of a hepatic artery infusion catheter for chemotherapy may require CT arterial portography to better demonstrate the arterial anatomy and lesions; however, the quality of CT and MRI has improved to such a degree that CT arterial portography is less commonly performed. Because aberrant vessels may course laterally and more posteriorly in the porta hepatis, dissection of the porta hepatis may be modified if an anatomic anomaly is noted preoperatively.
Intraoperative Ultrasound
Intraoperative ultrasound of the liver can function as both a diagnostic and a therapeutic tool. As a diagnostic aid, intraoperative ultrasound has proved successful for the following indications: to detect small (1-5 cm), nonpalpable, parenchymal liver lesions not detected by preoperative imaging studies; to precisely define the topography of liver tumors and their relationship to the vessels, allowing segmental resections to be performed; to guide intraoperative biopsy or ablative treatments of impalpable lesions; and to differentiate liver neoplasms from certain benign lesions, such as cysts and hemangiomas ( Figs. 91-15 and 91-16 ). Several studies have documented the utility of this technique, particularly with its initial use. In the study of Conlon and coworkers, the information provided by intraoperative ultrasound changed the preoperative surgical plan in 18% of patients and provided additional useful information not available preoperatively in 47%, including detection of subcentimeter lesions, lesion characterization, and anatomy of hepatic vasculature. In another study, the sensitivity of intraoperative ultrasound for lesion depiction was 94.3% compared with 86.7% for MRI. However, in this study, in only 4% of the patients did intraoperative ultrasound alter surgical management secondary to additional lesion detection. Another study demonstrated intraoperative ultrasound to change surgical management in only 7% of patients. Wagnetz and colleagues compared intraoperative ultrasound with 1.5T MRI and 64-MDCT and found that the sensitivity of ultrasound for lesion detection was 95.1% compared with 96.8% for MDCT and 94.4% for MRI. Furthermore, MDCT and MRI had a slightly higher negative predictive value than intraoperative ultrasound for identifying disease-free segments in the liver. In only 2.7% of cases was management changed after intraoperative ultrasound. An article by Mui and associates evaluating intraoperative sonography in detection of liver metastases in pancreatic adenocarcinoma found that ultrasound changed management in only 1% of cases. In comparison to initial studies, these more recent studies demonstrate a decreased percentage of cases in which intraoperative ultrasound alters surgical management. This is thought to be partly attributed to technologic advances in preoperative studies such as MRI, resulting in improved selection of patients.


Recent interest has arisen in the use of intraoperative contrast-enhanced ultrasound for liver lesion detection, citing increased lesion detection rates relative to noncontrast ultrasound and CT. Despite somewhat conflicting data, intraoperative ultrasound continues to be an important component of hepatic resection for purposes of lesion localization and relationship to hepatic vasculature and biliary structures, segmental localization, and further evaluation for incompletely characterized subcentimeter lesions identified on preoperative imaging studies. Intraoperative ultrasound also helps detect occult extension of hepatic tumors into vascular structures and assists in intraoperative biopsy of liver lesions. Intraoperative ultrasound has also proved useful in guiding intraoperative cryotherapy and radiofrequency ablation in the treatment of liver tumors.
Indications
As surgical techniques become safer, the indications for hepatic surgery are broadening (see Table 91-3 ).
Benign Lesions
The indications for resection of a benign tumor or cyst include risk of rupture or hemorrhage, symptoms, risk of malignant transformation, and enlargement of the mass so that it is causing pressure on bile ducts, the liver capsule, or adjacent organs. Fenestration-resection is rarely required for cosmetic or symptomatic improvement in patients with adult polycystic liver disease.
Localized biliary abnormalities, such as hepatolithiasis and segmental Caroli’s disease, are other indications for resection. Hepatic abscesses that do not respond to medication or percutaneous drainage may also require surgical resection.
Malignant Lesions
Resection of liver metastases is the most common indication for partial hepatectomy in the United States. Liver metastases are an enormous clinical problem in view of the fact that approximately 25% of all malignant neoplasms eventually spread to the liver ( Table 91-4 ). It is the most common site of blood-borne metastasis and more often than not is a major contributor to the patient’s demise. Neoplasms grow five to seven times faster in the liver than in most other organs. This is precisely why untreated liver metastases augur such poor survival. Colorectal metastasis is the most commonly resected hepatic tumor in the United States. Five-year survival rates of 25% to 40% for solitary metastases confined to one lobe have been reported. In a recent meta-analysis, the overall median survival for all colorectal metastases resected was 3.6 years. Hepatoma is the most commonly resected tumor worldwide owing to its prevalence in portions of the Far East and Africa. Technical improvements in hepatic resection and earlier diagnosis have increased the resectability rates. Patient selection is paramount in the success of hepatic resection for hepatocellular carcinoma. In fact, according to the Barcelona Clinic Liver Cancer group staging system, which has been integrated into the American Association for the Study of the Liver guidelines, hepatic resection has a marginal role in the treatment of hepatocellular carcinoma and is indicated in patients with early-stage hepatocellular carcinoma. Early-stage hepatoma is defined by the Milan criteria, normal clinical performance status, and preserved liver function. Liver resection in such patients (as well as higher stage) yields favorable 3- and 5-year survival rates of 78% and 39%.