Fig. 36.1
Thyroid monopolar RFA system. The patient is part of a closed-loop circuit that includes a radiofrequency generator, an electrode needle for ablation, a pump for cooling the electrode needle, and a large dispersive electrode (ground pads). Upper left: multiple ellipsoid ablation areas around are obtained by moving the active tip of electrode needle (moving shot technique)
36.2.2 Devices
For monopolar thyroid RFA straight, internally cooled, short (7- or 10-cm), thin (18- or 19-gauge) electrode needles are currently used. The active tips of the electrode needles may have a different length (0.5–2.0 cm) according to the desired ablation area [3, 4]. Experience with bipolar radiofrequency electrode needles has not been reported for thyroid ablation. In the past other types of electrode needles (e.g., multi-tined expandable electrodes) had been used for thyroid RFA , but their use is no longer recommended [5–7].
36.2.3 Technique (See Video 36.1)
Thyroid RFA is performed as an outpatient procedure in an interventional suite. Patients should be fasting. The patient is placed on an operation bed in the supine position with hyperextended neck, and a venous catheter is inserted in a forearm vein. A multiparametric monitor is connected to the patient displaying a continuous single lead electrocardiogram, pulse oximetry, blood pressure, and respiratory rate. Conscious sedation is obtained with intravenous (i.v.) midazolam (2–5 mg) in fractionated boli in order to decrease the patient’s anxiety, swallowing, cough, and movements. Local anesthesia with 2 % ropivacaine pericapsular infiltration (2–5 mL) is performed under US assistance with thin (27-gauge) needles.
In the RFA monopolar technique, the patient is part of a closed-loop circuit: ground pads (dispersive electrodes) applied to both thighs are connected to a radiofrequency generator and the generator is connected to an electrode needle. These electrode needles are straight, internally cooled, 7-cm, 18-gauge and have active tips of usually 1.0 cm for most thyroid nodules.
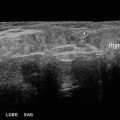
The ablation is performed according to the “moving shot” technique: the nodule is divided into multiple conceptual areas and thereafter the nodule is ablated unit-by-unit by moving the electrode tip (Fig. 36.2) [1, 8–10]. The ablation starts from the deepest portion of the nodule with a transisthmic approach. The needle is inserted transisthmically at the higher level according to patient anatomy. This insertion allows the repositioning of the needle as much as 60–90° with respect to the entry point in order to achieve ablation of more medially located tissue. This maneuver does not increase side effects, particularly vocal cord palsy, because while continuously tilting the probe, careful surveillance of the area medial to the needle is required to detect US signs of heating (gas microbubbles) when the needle is inserted close to the tracheoesophageal groove where the inferior laryngeal nerve runs (the so-called “danger triangle”). When a hyperechoic area appears and when impedance increases the tip is moved backward to an untreated more superficial area. The maneuver is repeated with repositioning the needle, until all areas are ablated (Fig. 36.3). Usually RF power starts with 30 W and 5-W upward adjustments are made up to 60 W. Higher RFA power is used in Korea [11]. The successful ablation of a unit is confirmed by the appearance of a hyperechoic area—due to microbubbles—and the abrupt increase of impedance (the so-called “break point”) registered on the RF generator monitor.



Fig. 36.2
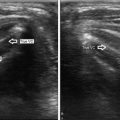
The moving shot technique. According to the moving shot technique the electrode needle delivers energy and a hyperechoic area is seen along the track of the needle (sagittal view, B-mode)

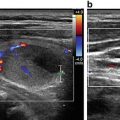
Fig. 36.3
A large (33 mL) spongiform thyroid nodule before RFA (a) and immediately after the procedure in B-mode (b) and with color Doppler (c). After RFA the nodule is unstructured and not vascularized. The color spot of (c) (lower left) is the common carotid artery
To treat mixed nodules, the cystic fluid is first aspirated and then the ablation is performed. However, sometimes, bleeding occurs during cyst aspiration. To control the bleeding, 99 % ethanol can be injected into the evacuated cystic cavity [12]. The injected volume of ethanol corresponds to approximately 50 % of the aspirated fluid volume. Ethanol causes direct coagulative necrosis and local small vessel thrombosis, stopping the bleeding. After 2 min of ethanol retention, as much of the injected ethanol as possible is removed, and RFA may finally start.
Immediately after the RFA procedure, methyl-prednisolone 20 mg i.v. bolus may be administered in order to prevent pain. Patients are then brought to the recovery room and are kept under observation for 2 h. The day following the RFA procedure, patients can be started on oral methyl-prednisone taper of 16 mg daily for 5 days, 8 mg daily for 4 days, and 4 mg daily for 3 days. Oral proton pump inhibitors are administered for 12 days [10].
36.2.4 Indications
RFA can be used to treat [3, 4]:
Nonfunctioning, benign, solid or predominantly solid, thyroid nodules in patients presenting with local symptoms or cosmetic complaints when surgery is contraindicated or declined.
Autonomously functioning thyroid nodules (AFTNs ) causing problems related to thyrotoxicosis when surgery or radioiodine are contraindicated or declined.
Recurrent thyroid cancers located in the thyroid bed as well as metastatic lymph nodes when surgery is contraindicated and radioiodine is ineffective.
RFA is not recommended for the treatment of primary thyroid cancers because there is no evidence of its efficacy for malignancy. Similarly it is not recommended for treatment of nodules with follicular neoplasm cytology since, about 15–30 % of cases labelled as follicular neoplasm prove to be malignant [13].
36.2.5 Clinical Results in Benign Cold Thyroid Nodules
At present, the most important indication for RFA in the thyroid gland is to reduce the volume of benign cold thyroid nodules in patients with local pressure symptoms or cosmetic complaints. Benignity of thyroid nodules is usually confirmed by at least two separate fine needle aspiration (FNA) biopsies. The first study suggesting the use of RFA to treat symptomatic thyroid nodules was published in 2006 [17]. Since then, many studies have confirmed the efficacy and safety of treating benign thyroid nodules with RFA . Recently, two systematic reviews with meta-analyses have confirmed that RFA is effective in both reducing the volume of thyroid nodules and improving the related compressive symptoms and aesthetic problems, without causing thyroid dysfunction or serious complications [18, 19]. Moreover, RFA of symptomatic thyroid nodules improves patients’ health-related quality of life [10]. Some authors have found that the efficacy of thyroid RFA is similar to that of thyroid surgery [20, 21].
The reduction in thyroid nodule volume after RFA ranges from 50 to 90 % [22–28]. The heterogeneity of results among studies can be explained by a number of factors: (a) different types of electrodes used; (b) a single treatment versus repeated RFA sessions; (c) different initial volume of treated nodules; (d) different composition (solid, cystic, or mixed) of the target nodules. Nodule volume reduction is gradual and final results are achieved approximately 1 year after RFA or even more in case of large lesions (Fig. 36.4). The goal of thyroid RFA is to perform the procedure only once. Additional ablation can be performed if results are partial or in case of thyroid nodule regrowth [29–31]. In a 4-year follow-up study thyroid nodule regrowth was recorded in only 5.6 % of the patients (7/126) [31]. Importantly, RFA does not affect eventual subsequent thyroid surgery [32].


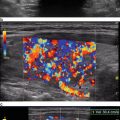
Fig. 36.4
US follow-up images of a mixed thyroid nodule treated with RFA . The nodule had a volume of 10 ml (a). Before RFA application the fluid component was drained. The day after RFA there was no fluid component and the nodule measured 7.4 ml (b). After 6 months the nodule measured 1.2 ml (c). After 1 year the nodule had shrunk to 0.65 ml (d). The ablated nodule shrinks over time and becomes inhomogeneous and hypoechoic
RFA does not alter the thyroid function [10]. This is also true for extended thyroid tissue thermal ablation. Indeed, in patients with previous lobectomy, RFA does not affect blood thyroid hormone levels [33]. Similarly, the thyroid function of patients treated with RFA for bilateral benign nodules is also preserved [34]. We need to keep in mind that an ablated thyroid nodule becomes hypoechoic, inhomogeneous, and avascular on color Doppler ultrasonography [35]. Therefore, it is important not to confound a treated thyroid nodule with a thyroid cancer.
Ethanol ablation (EA) is the first-line treatment for benign, symptomatic, cystic (cystic portion >90 %) or predominantly cystic (cystic portion less than 90 % and greater than 50 %) thyroid nodules [36–38]. Notwithstanding, RFA is also effective in treating cystic or predominantly cystic thyroid nodules, but is more expensive than EA and requires more sessions [39–41]. Despite the effectiveness of EA, some patients may have unsatisfactory results, mostly because of the solid component of the nodule which does not respond to alcohol. In this case, RFA has been used successfully to solve persistent clinical problems after an EA session [42, 43].
36.2.6 Clinical Results in Benign Autonomous Functioning Thyroid Nodules
The first case report of the successful treatment of an autonomous functioning thyroid nodule (AFTN ) was published in 2008 [44]. Subsequently, additional reports demonstrate that RFA is effective in patients with AFTNs since it reduces the nodule volume and controls hyperthyroidism [6, 8, 45]. RFA ablation of AFTNs requires a complete ablation, as untreated peripheral areas tend to regrow, causing relapse of hyperthyroidism. It has been shown that for the treatment of AFTNs more than one RFA session may be necessary. However, there are no studies comparing RFA to radioiodine for the treatment of AFTNs .
36.2.7 Clinical Results in Recurrent Thyroid Cancer
Occasionally well-differentiated thyroid cancer (DTC ) may recur, mainly in the thyroid bed or in lymph nodes [46]. In this case, surgical removal is recommended, sometimes followed by radioiodine [38]. However, repeated neck dissection is difficult due to distortion of normal tissue planes by scar tissue formation, and such operations are associated with a higher rate of complications such as recurrent laryngeal nerve injury, hypoparathyroidism, and skin scar formation [47]. In 2001, RFA was proposed as a treatment for regional recurrence from DTC [48]. Subsequently, other studies have also confirmed the efficacy of RFA for treating thyroid cancer recurrences to cause volume reduction or complete disappearance of tumor in some cases, decrease of serum thyroglobulin concentrations, and improvement of clinical symptoms if present. The RFA technique used for the treatment of DTC recurrences is similar to that used for benign thyroid nodules, but has additional precautions. First, the lesion has to be confirmed to be malignant by US-guided FNA biopsy and FNA thyroglobulin measurement. Second, since these lesions are usually small, use of electrode needles with a shorter active tip (i.e., 0.5 or 0.7 cm) is generally more appropriate. RFA starts with low power (5 W) and the power is gradually increased until a hyperechoic area forms. Thermal damage to nerves, especially recurrent laryngeal nerve and vagus nerve, during RFA treatment is of more concern than damage to blood vessels, as blood flow within the vessels dissipates the heat (the so-called heat sink effect). On the other hand, there is the possibility that the heat sink effect might reduce RFA efficacy if treating lesions close to a large vessel (common carotid artery or internal jugular vein) because of the cooling effect of blood flow. In order to avoid thermal injury of neck nerves during RFA, sterile water or 5 % dextrose solution can be injected with a 23-gauge needle between the expected location of these vital structures and the tumor (hydrodissection technique). Thus, a protective barrier displaces the target from critical structures (not only nerves, but also trachea and esophagus). Saline solution is not recommended for hydrodissection in RFA because it conducts electricity. During hydrodissection technique, if a continuous infusion of fluid is deemed necessary, the tip of the hydrodissection needle should be placed at least 1 cm away from the tip of the RF applicator. Another strategy that prevents thermal injury is the use of the electrode needle as a lever to increase the distance between tumor and critical structures. Using this technique, tumor is pulled away from vital structures by tilting the electrode needle during ablation under continuous US guidance [49–56].
36.2.8 Complications of Thyroid Radiofrequency Ablation
Various complications may occur during thyroid RFA . A large multicenter Korean study reported an overall complication rate of 3.3 % associated with the RFA treatment of benign thyroid nodules [57]. In order to minimize complications, it is important to have a thorough knowledge of sonographic anatomy of the neck [58].
Intraoperative pain is usually well controlled by means of local anesthesia with ropivacaine and conscious sedation with midazolam [10]. Notwithstanding, in case of intense pain, local or radiating to the jaw, teeth, chest, or back, the RF generator is turned off. Subsequently, the electrode needle is repositioned in a more central area of the nodule and the ablation can proceed. Intranodular bleeding during needle insertion may occur and is seen as a rapidly expanding hypo/anechoic signal within nodular tissue. It can be stopped by swift needle-electrode insertion and heat administration. Intranodular bleeding does not prevent ablation procedure. Thyroid pericapsular bleeding is seen as a hypoechoic layer surrounding the thyroid. Pericapsular hematomas can be controlled by compression of the neck for a few minutes. Neck bruising may follow a few days later, and it disappears in about 3–4 weeks. A rarely reported complication is vasovagal reaction which presents with bradycardia, hypotension, vomiting and defecation. If this reaction is observed, the bed is tilted in Trendelenburg position and maneuvers are temporarily interrupted until spontaneous recovery, which occurs in a few minutes. Vasovagal syncope is due to vagus nerve stimulation as the nodule may displace the common carotid artery and the internal jugular vein. For this reason, knowledge of cervical vagus nerve localization is of paramount importance in thyroid RFA [59]. Cough during thermal ablation is due to trachea stimulation and the tip of the needle should be pulled back.
Nodule rupture presents with sudden neck bulging and pain during the early follow-up period (2–4 weeks) [60]. The rupture might occur due to volume expansion because of intranodular bleeding. An initial conservative treatment with compression is recommended. Another cause for tumor rupture may be liquefaction which occurs typically 2–4 weeks after RFA procedure. However, in case of deterioration of symptoms, drainage or excision may be required. Infection or abscess formation is rare, since the skin is sterilized with povidone–iodine solution. Skin burns have been reported at the electrode needle puncture site. Application of an ice bag during ablation or injection of fluid between the nodule and skin may prevent skin burns. Voice change is a serious complication of thyroid RFA which is due to thermal injury of the recurrent laryngeal nerve. This phenomenon may be prevented by undertreating the area near the danger triangle (i.e., the tracheoesophageal groove which contains the recurrent laryngeal nerve). Finally, RFA may cause the new appearance of thyroid antibodies (anti-thyroglobulin, anti-thyroid peroxidase, or anti-thyrotropin receptor antibodies), but no subsequent clinical consequences (hypothyroidism or hyperthyroidism) have been reported.
36.2.9 Comparison Between RFA and Other Nonsurgical Techniques for Benign Thyroid Nodules
There are no head-to-head comparisons between RFA and other US-guided nonsurgical techniques for the treatment of benign thyroid nodules. However, a recent systematic review including traditional pooling and Bayesian network meta-analysis found that RFA is superior to laser ablation in reducing benign solid thyroid nodule volume, despite the smaller number of treatment sessions without major side effects [61]. It is clear that interventional thyroidology, and especially RFA , is playing an increasing role in the management of thyroid nodule pathology [62].
36.3 High-Intensity Focus Ultrasound
36.3.1 Device and Technique
High intensity focused ultrasound (HIFU ) ablation is an extracorporeal thermal treatment that is used in the endocrine neck diseases for treating benign thyroid nodules (cold or hot) and parathyroid adenomas or hyperplastic parathyroids since 2010. High intensity ultrasound beam, as opposed to the low energy field used for ultrasound imaging, when absorbed by tissue, creates a rise in temperatures (approximately 80–95 °C) with subsequent nodular coagulation and cavitation. The ultrasound beam is focused, and for this reason the tissue thermal injury is confined only to a well delimited area, minimizing the effects on surrounding structures. At the present time, the only available system for HIFU treatment of thyroid and parathyroid lesions is the EchoPulse (Theraclion, Paris, France). The EchoPulse device is composed of a mobile electronic unit with energy generator, a robotic articulated arm with a visualization and treatment unit (VTU) and a touch-screen monitor for ablation planning and follow-up (Fig. 36.5). The VTU contains both the ultrasound imaging transducer (7.5 MHz) and the HIFU transducer (3 MHz) for delivering the energy to the target (Fig. 36.6). A cooling circuit specific for the device lowers the temperature between consecutive pulses. The HIFU pulse produces an ablation area of 9 mm in length and a 2-mm diameter. After manually drawing the area to treat on the touch screen monitor, multiple pulses are automatically delivered to carry out the ablation. The safe margins of the treated area are 3 mm from trachea, 2 mm from carotid artery, and 5 mm from skin. The maximum treatable depth from the skin surface is 28 mm. The procedure is usually performed under conscious sedation and seldom local anesthesia is required.



Fig. 36.5
The EchoPulse HIFU device is composed of an electronic mobile unit with energy generator, a robotic articulated arm with a visualization and treatment unit (VTU) , and a touch-screen monitor for ablation planning and follow-up

Fig. 36.6
The VTU contains both the ultrasound imaging transducer (7.5 MHz) and the HIFU transducer (3 MHz) for delivering the energy to the target. A cooling circuit specific for the device turns down temperature among consecutive pulses
36.4 Clinical Results
Initial HIFU experimental studies on animal thyroid ablation were published in 2004 [63] and 2009 [64]. The first case report of a successful HIFU ablation of a human AFTN was published on 2010 [65]. Since then, a few studies of a relatively limited number of patients have assessed the HIFU treatment efficacy and safety profile for the ablation of cold and hot thyroid nodules [66–71] and of parathyroid lesions linked to primary and secondary hyperparathyroidism [72–75]. The results of these studies are encouraging, but the HIFU procedure still carries some limiting factors. The procedure is time consuming. The patient has to remain still during the entire ablation time since if he or she moves, the treatment planning has to be restarted from the beginning. In addition more treatments are necessary to treat large lesions and deep lesions cannot be ablated since the maximum treatable depth is only 28 mm from the skin. If there are skin scars or moles between the probe and the skin, HIFU ablation is not possible. Larger studies are needed to integrate HIFU ablation of neck diseases into clinical practice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree