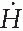High-Dose-Rate Brachytherapy
23.1. INTRODUCTION
Most of the clinical experience in brachytherapy has been obtained using low-dose-rate (LDR) implants, that is, with prescription dose rate on the order of 0.5 to 2 cGy/min. The International Commission on Radiation Units and Measurements (ICRU) Report 38 (1) classifies high dose rate (HDR) as 20 cGy/min or higher. With the introduction of remote afterloading technology, it is possible to deliver HDR brachytherapy safely and more precisely than possible with the classical LDR brachytherapy. Although LDR brachytherapy can also be delivered using remote afterloading devices, logistic problems of prolonged treatment and patient hospitalization make LDR less attractive than HDR.
As discussed in Chapter 15, the principal advantage of HDR over LDR is that it permits treatments on an outpatient basis. For that reason, it is well suited for treating large patient populations. Greater control over dose distribution is another major advantage, which is being used for delivering highly conformal dose to well-localized tumors, for example, as a boost or primary treatment for prostate cancer. Although the role of HDR in brachytherapy is not yet fully established, all indications point toward its widespread use as a sole procedure or in conjunction with external beam. If the current trends continue, it is quite possible that HDR will replace all brachytherapy techniques in the not too distant future.
23.2. HIGH-DOSE-RATE UNIT
A. REMOTE AFTERLOADER
An HDR remote afterloading unit contains a single source of high activity (˜10 Ci or 370 GBq). Although cobalt-60 and cesium-137 have been used in the past, iridium-192 is the most commonly used radioisotope in HDR. For HDR brachytherapy, 192Ir is the best choice because of its higher specific activity (allows smaller source for the same activity) and lower photon energy (requires less shielding). A disadvantage, on the other hand, is its shorter half-life, necessitating source replacement every 3 to 4 months.
The 192Ir source used in HDR is a small line source welded to the end of a flexible drive cable. The cable with the source attached at the end is also called source wire. The dimensions of the source vary between 0.3 and 0.6 mm in diameter and 3.5 and 10 mm in length, depending on the HDR model. The source wire, when not extended, is stored in a shielded safe of the HDR unit (Fig. 23.1A). In compliance with the NRC regulations (2), the leakage radiation levels outside the unit do not exceed 1 mR/h (0.01 mSv/h) at a distance of 10 cm from the nearest accessible surface surrounding the safe with the source in the shielded position.
The HDR unit is equipped with several channels and an indexer system to direct the source to each channel. In some models, channels are provided on a rotating turret in which any channel can be aligned with the source wire path (Fig. 23.1B). Applicators or catheters implanted in the patient are connected to the channels by catheters called transfer tubes or transfer guides. Before the active source wire is extended for treatment, a dummy wire is extended to verify that the path is clear of any obstruction.
The source wire (or dummy wire) can be advanced or retracted through individual channels, transfer tubes, and applicators by a remote computer-controlled drive mechanism consisting of stepper motors. The positioning of the source at the programmed dwell positions in the applicators is accomplished in precise increments by the stepper motors. The positioning accuracy of the source is specified at ±1 mm. The dose control precision is provided by a 0.1-second dwell time resolution.
A number of safety systems are provided for the HDR. For example, interlocks prevent initiation of treatment if the door is open, the applicator is not attached, or the connections between a programmed channel, the transfer tube, and the applicator are not secure. Backup batteries are provided to take over operation in case of power failure. A manual source retraction mechanism is available to withdraw the source into the storage safe if it gets stuck and cannot be retracted by the emergency switch. The treatment is aborted if the system detects blockage or excessive friction during source transit.
B. HIGH-DOSE-RATE APPLICATORS
Brachytherapy applicators used for LDR implants can also be used for HDR. For example, some of the most commonly used applicators, for a variety of HDR applications, are Fletcher-Suit or Fletcher-Suit-Delclos. These applicators are used for the treatment of gynecologic malignancies of the uterus, cervix, and pelvic side walls. The applicator set typically consists of three rigid intrauterine tandems, with curvature of 15-, 30-, and 45-degree angles, and a pair of ovoids or colpostats with shields in place to reduce dose to rectum and bladder.
Vaginal Cylinder. These are acrylic cylinders having a variety of diameters and axially drilled holes to accommodate a stainless steel tandem. Coupling catheters for attachment to the transfer tubes and marker wires to fit the length of the tandem are provided in the set. The applicator is suitable for treating tumors in the vaginal wall.
Rectal Applicator. Acrylic cylinders of different diameters are designed to treat superficial tumors of the rectum. Selective shielding is incorporated to spare normal tissue. Coupling catheters and marker wires are provided in the HDR set.
Intraluminal Catheter. Suitable diameter catheters of various lengths are available for treating intraluminal disease such as endobronchial carcinoma.
Nasopharyngeal Applicators. These applicators are used for treating nasopharyngeal tumors with HDR. The applicator set includes a tracheal tube, catheter, and nasopharyngeal connector. Besides the above examples, HDR applicators and catheters are available for virtually every type of application deemed suitable for intracavitary brachytherapy.
Interstitial Implants. Hollow, stainless steel needles are implanted into the tumor following standard brachytherapy rules of implant (see Chapter 15) and closed-ended catheters are inserted to accommodate the HDR source wire. Marker wires are used to plan the dwell positions of the source as with the other HDR applicators. Examples of interstitial implants are prostate gland, breast, and some head and neck tumors.
Breast Balloons. These applicators are used for treating breast cancer after lumpectomy. The applicator consists of one or more catheters placed inside a saline-filled balloon which is implanted within the lumpectomy cavity. The HDR source is positioned to treat the PTV, which consists of a 1-cm rind surrounding the balloon.
C. FACILITY DESIGN
C.1. Shielding
The HDR unit must be housed in an adequately shielded room. The shielding and safety requirements are mandated by the Nuclear Regulatory Commission (NRC) (2). The HDR treatment room can be designed as a dedicated facility (e.g., “HDR suite”) or adopted from an existing 60Co or linac room. In either case, the shielding must satisfy or exceed the NRC requirements.
The shielding calculations are based on the dose limits specified by the NRC in 10 CFR 20.1301 (for individual members of the public) and 10CFR 20.1201 (for occupational personnel). The NRC annual effective dose equivalent limits follow the National Council on Radiation Protection and Measurements (NCRP) guidelines (see Table 16.5). These are summarized as follows:
Public: 0.1 rem (1 mSv) in 1 year for continuous or frequent exposures; or 0.5 rem (5 mSv) in 1 year for infrequent exposure. In the case of HDR, the limit for infrequent exposure, namely, 0.5 rem in 1 year, is more relevant.
Occupational: 5 rems (50 mSv) in 1 year.
In addition to the annual limits, the NRC requires that the dose in any unrestricted area must not exceed 2 mrem (0.02 mSv) in any 1 hour. The italicized words mean that with the workload and use factor applied, the dose received in an unrestricted area shall not exceed 2 mrem in any 1 hour.
The methods of calculating primary and secondary barriers are the same as discussed for megavoltage beams in Chapter 16. Equations 16.4, 16.6, and 16.10 are valid also for HDR room design, provided appropriate factors related to 192Ir source are used. These factors include average photon energy = 0.38 MeV, 10th-value layer (TVL) = 5.8″ of concrete (density 2.35 g/cm3), and exposure rate constant = 4.69 R-cm2/mCi-h. The following examples illustrate the method of barrier thickness calculation or evaluation of an existing barrier.
EXAMPLE 1
Calculate the barrier thickness at a distance of 5 feet from the HDR source to protect a controlled area.
Because the HDR source, 192Ir, requires less shielding than a megavoltage teletherapy unit and can be assumed isotropic (same intensity in all directions) in the context of shielding design, it is reasonable to construct all barriers of the same thickness. Additionally, as a conservative measure, one could design all barriers as primary, for a maximum transmission of 2 mrem in any 1 hour; or, even more conservatively, a limit of 2 mrem/h (instantaneous dose rate) could be adopted.
Assuming that, from the radiation protection point of view in this case, 1 R ≅ 1 cGy ≅ 1 rem, the dose equivalent rate (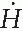 ) at a distance of 5 feet from the source is given by (inverse square law)
) at a distance of 5 feet from the source is given by (inverse square law)
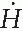 ) at a distance of 5 feet from the source is given by (inverse square law)
) at a distance of 5 feet from the source is given by (inverse square law)B = 10-3
If n is the number of TVLs required for shielding,
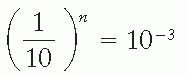
or
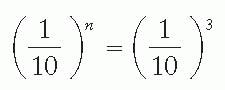
or
n = 3
Since TVL = 5.8″ of concrete (3):
Barrier thickness = 3″ × 5.8″
= 17.4″
Thus, an HDR suite in this case would have all barriers (walls, floor, and ceiling) of thickness about 18″ of concrete provided a minimum clearance, 5 feet in this case, between the source and the area to be protected is assured. These shielding requirements could be reduced if a larger clearance is maintained and realistic workload and occupancy factors are applied.
If no maze is provided to prevent direct incidence of radiation beam at the door, the door must be shielded (e.g., with lead lining) for a transmission equivalent to that of 18″ of concrete or 3 TVLs. Because the TVL for 192Ir γ rays in lead is 2 cm (3), the equivalent lead thickness must be 2 × 3 = 6 cm. A better alternative is to provide a maze or add a shielded partition between the source and the door.
EXAMPLE 2
What is the door shielding required if a maze is provided for an HDR suite, with source to wall (facing the door) distance of 15 feet and maze length of 10 feet?
With the maze wall thickness of 18″ of concrete, the transmitted dose incident at the door would be 2 mrem/h or less. The dose due to scatter off the wall facing the inside of the door can be calculated as follows.
Assuming average reflection coefficient (α) of 2 × 10-2/m2 for 192Ir (4) and the area of scatter at the facing wall to be 5 m2, the dose rate (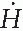 s) at the inside of the door due to scattering would be
s) at the inside of the door due to scattering would be
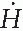 s) at the inside of the door due to scattering would be
s) at the inside of the door due to scattering would bewhich is negligible. Thus, no shielding is required for the door if an appropriate maze is provided in an HDR suite.
EXAMPLE 3
Evaluate the shielding of an existing 6-MV linear accelerator for HDR use.
Transmitted dose for each existing barrier can be calculated using the inverse square law and TVL for 192Ir γ rays, as discussed above. For example, if a secondary barrier for a 6-MV room is 40″of concrete and the minimum distance between the source and the area to be evaluated for protection is 10 feet, then the effective dose equivalent rate (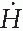 ) in the area can be calculated as follows:
) in the area can be calculated as follows:
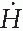 ) in the area can be calculated as follows:
) in the area can be calculated as follows:
which is negligible. Similar calculations have shown that rooms designed with adequate shielding for megavoltage teletherapy units are more than adequate for HDR shielding (5). Whether it is a dedicated HDR suite or an existing teletherapy vault, the shielding adequacy of the facility must be documented before applying for an HDR license. Since most institutions use existing teletherapy rooms to house HDR units, a shielding evaluation report must be submitted with the license application as required by the NRC.
C.2. Safety Features
Safety requirements for a dedicated HDR vault or an existing teletherapy room adopted for HDR are mandated by the NRC (2) or state. These include electrical interlock system that retracts the source when the door is opened and does not allow resumption of the treatment unless the door is closed and the interlock is reset; mechanism to ensure that only one device can be placed in operation at a given time if the HDR is installed in an existing teletherapy room; inaccessibility of console keys to unauthorized persons; a permanent radiation monitor capable of continuous monitoring of the source status; continuous viewing and intercom systems to allow for patient observation during treatment; and restricted area controls such as signs, locks, visible/audible alarms, and door warning lights indicating “Radiation On.”
23.3. LICENSING REQUIREMENTS
Purchasers of HDR units must apply for a license or license amendment with the appropriate regulatory agency. In the United States, it is the NRC or the state if it is an Agreement State. The licensing requirements for LDR brachytherapy were discussed in Chapter 16, Section 16.10. Essential items included (a) the applicant’s qualifications (education, training, experience) and a description of the personnel training program (initial as well as periodic); (b) administrative requirements: ALARA program, radiation safety officer, radiation safety committee, and a written quality management program; and (c) technical requirements: calibration and survey instruments, leak testing and inventory of sources, conditions for patient release, posttreatment
survey of patients, and posting of radiation signs. If the applicant already has an LDR brachytherapy license, an amendment must be requested for the remote afterloading device by listing license conditions specific to the use of that device.
survey of patients, and posting of radiation signs. If the applicant already has an LDR brachytherapy license, an amendment must be requested for the remote afterloading device by listing license conditions specific to the use of that device.
The information required specifically for HDR licensing is contained in the NRC document (2).
A typical license application includes the following information:
Source description (radionuclide, manufacturer’s name and model number, maximum activity, maximum number of sources to be possessed at the facility at any given time, and the physical construction of the source)
Manufacturer’s name and model of the HDR unit
Intended use (cancer treatment in humans using interstitial, intracavitary, intraluminal brachytherapy, etc.)
Authorized users (physicians) and authorized physicist(s), verifying that they meet educational and experience qualifications set forth in 10 CFR 35.690
An outline of initial training of authorized users and device operators (didactic training plus a minimum of 8 hours of “hands-on” device operation training)
Description of radiation detection and survey instruments to be used
A floor plan of the facility, identifying room(s); doors; windows; conduits; density and thickness of shielding materials of walls, floors, and ceiling; and distances to the adjacent inhabitable areas with indication of whether the areas are restricted or unrestricted
Shielding calculations to show that the adjacent areas comply with the regulatory standards
Area security and safety features (Section 23.2 C.2)
Calibration procedures and frequency, leak test procedures and frequency, and the qualifications of those performing these tests
Quality assurance program, including pretreatment or daily quality assurance procedures and periodic testing (e.g., monthly, quarterly, annually)
Training and frequency of retraining of individual operators (e.g., annually or every 2 years)
Training or certification of individuals performing source changes (normally vendor representative)
Personnel radiation monitoring program
Emergency procedures, postings, and locations
Disposal arrangements of decayed sources (usually by return to vendor)
Operating procedures and manuals, their availability to personnel, and location
Inspection and servicing of HDR equipment at intervals not to exceed 1 year, by the manufacturer or a person licensed by the NRC/Agreement State; records of inspection and service to be maintained for the durations of the license for guidance
Note: Requirements of license application may change from time to time. The applicant should consult the most current NRC document.
A. HIGH-DOSE-RATE POLICIES AND PROCEDURES
As a condition for awarding an HDR license, the NRC requires a written procedure as it does for the LDR brachytherapy. General requirements of such a program were discussed in Chapter 16, Section 16.10B. Written policies and procedures include general as well as specific tests to ensure safe application of the HDR procedure. The program is designed by the institution and must be submitted as part of the license application. A sample HDR program is described below.
A.1. Written Directive
A written directive (prescription) must be provided by the “authorized physician user” that includes the name and hospital number of the patient, the HDR source material, the dose per fraction, the number of fractions, the total dose, and the site of administration.
A.2. Patient Identification
The identity of the patient must be verified by two independent methods as the individual named in the written directive. This may be accomplished by asking the patient his or her name and confirming the name by comparison with the patient’s identification bracelet or hospital identification card.
A.3. Treatment Plan Verification
The “authorized physician” and the “authorized physicist” must check the treatment plan to ensure that (a) the plan parameters (e.g., source specification, source strength, source position, dose per fraction, total dose) are correct and in accordance with the written directive; (b) the
treatment parameters generated by the plan for input into the HDR device (e.g., channel numbers, source positions, dwell times) are correct; and (c) the dose distribution agrees with an independent (e.g., manual) spot check within reasonable limits (e.g., ±5%).
treatment parameters generated by the plan for input into the HDR device (e.g., channel numbers, source positions, dwell times) are correct; and (c) the dose distribution agrees with an independent (e.g., manual) spot check within reasonable limits (e.g., ±5%).
A.4. Pretreatment Safety Checks
Pretreatment safety checks of the HDR equipment must be performed on any day that the HDR procedure is scheduled. A sample is provided in Section 23.3B.
A.5. Treatment Delivery
Prior to the initiation of treatment, the “authorized operator” (authorized medical physicist, dosimetrist, or radiation therapist) must verify that the name of the patient, the dose, the site of administration, and the times for each dwell locations are in agreement with the written directive and the approved treatment plan.
A.6. Posttreatment Survey
Immediately after each treatment, a survey of the afterloading device and the patient must be performed to ensure that the source has been returned to the fully shielded position. The survey will include connectors and applicator apparatus, the full length of the catheter guide tube, and the external surface of the device to ensure that the source is fully retracted. The patient shall be surveyed over the body surface near the treatment site before removing the patient from the treatment room.
A.7. Source Replacement and Calibration Check
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree