X-Rays and Urology
Some of the earliest medical research involving X-rays involved the investigation and exposure of the biliary and renal tracts; John Macintyre, a Scottish doctor, imaged a kidney stone using X-rays. He was able to make a diagnosis of this stone only after five different patients suspected clinically of having renal calculus were photographed with negative results, but on the sixth attempt, in a patient previously known to have renal calculi, he was able to obtain a picture of an obliquely placed elongated deposit within the silhouette of the kidney. He confirmed the diagnosis in the subsequent operation and reported the case in the 11 July 1896 issue of The Lancet [8].
Before 1895, the practice of urology was almost exclusively based on cystoscopy, itself a relatively new development, as well as on laboratory and physical examination. Röntgen’s discovery changed the world of urology forever. Dr. Henry W. Cattell, Instructor of Anatomy at the University of Pennsylvania, in the United States, wrote, “…the manifold uses to which Roentgen’s discovery may be applied in medicine are so obvious that it is even now questionable whether a surgeon would be morally justified in performing a certain type of operations without first having seen pictured by this rays the field of his work…” [9].
Adding Contrast
The first documented contrast study of the urinary tract was performed in 1897 by the French surgeon Théodore Tuffier. He passed a radiopaque catheter through the ureteral orifice in the bladder hereby outlining the course of the ureter [10].
The first ureteral catheters in use were radiolucent and mounted around a lead wire; this technique was subsequently replaced by making the ureteral catheters themselves radiopaque, by impregnating their walls with iron oxide. In 1914, the urologist Pasteau invented a catheter, which included a semiopaque centimeter scale, to localize stones precisely [11].
The search for better ways to visualize the urinary tract continued; next came the use of air as a contrast agent by Wittek, who succeeded in demonstrating cystolithiasis, thus giving birth to the air cystogram [12].
Replacing air as a contrast medium was the next step, and Wulff, in 1904, was the first to employ a radiopaque solution composed of 10 % bismuth subnitrate and starch, filling what was in all likelihood a huge diverticulum as well as the bladder itself [13].
This solution was soon replaced by a different liquid contrast agent containing a colloidal suspension of silver, giving better image quality, and, by injecting larger quantities of solution into the bladder, delineating the ureters and renal pelvises, giving birth to the first retrograde pyelograms [14]. The usefulness of this technique was quickly recognized but, unfortunately, so were the dangers associated with the silver-containing contrast agent. The search for safer materials began, and sodium iodide solutions, first described by Cameron in 1918, [15] became the contrast agents of choice for retrograde pyelography.
The next step in this evolutionary process was to eliminate the need to directly introduce the contrast agent into the urinary system. An indirect means might be faster and safer. The discovery of iodine as intravenous safe radio contrast agent was accidental. In the early 1920s, when iodine-containing compounds were used to treat syphilis, a team of workers at the Mayo Clinic, Earl Osborne (a syphilologist), Albert Scholl (a urologist), Charles Sutherland (a radiologist), and Leonard Rowntree (an internist), described the use of intravenous and oral sodium iodide to visualize the urinary tract. Osborne noticed that the urinary bladder was visible on radiographs of patients taking large doses of oral and intravenous sodium iodide for the treatment of syphilis. The visualization of the renal pelvis was poor, but the authors calibrated the dose of iodine against the urinary iodine concentration and the degree of bladder radioopacity, and thus they went on to perform the first successful clinical pyelogram. However, sodium iodine was far too toxic for clinical radiodiagnosis [16].
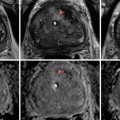
A few years later, in 1928, Moses Swick, while an intern at Mount Sinai Hospital in the Department of Urology, traveled to Hamburg, Germany, on a research scholarship to work with Professor Leopold Lichtwitz in the treatment of human biliary infections with the use of iodinated drugs. It occurred to Swick that these drugs, containing iodine, might be of value in visualizing the renal tract by radiography [17]. He made several studies in laboratory animals. The initial studies were very encouraging, and, in order to gain access to the large number of patients, Swick transferred his work to Berlin to the urological department of Professor Alexander von Lichtenberg. Consequently, the first successful human intravenous urography (IVUs) was produced using a soluble iodinated pyridine compound solution (Uroselectan) [18–20] (Fig. 1.2).
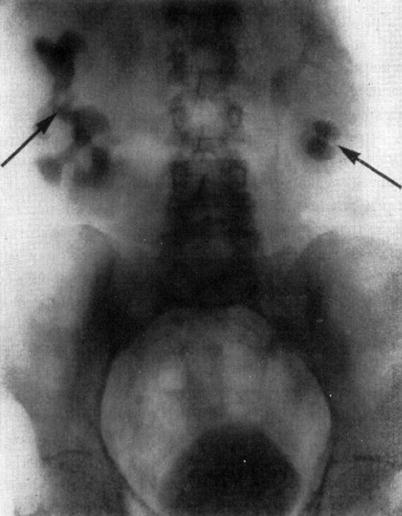

Fig. 1.2
Intravenous urography by Swick, (arrows) showing renal calyceal systems and bladder opacified (From Swick. [17] with permission)
In fact, iodinated pyridine compounds were routinely used to perform IV urography for the next 20 years.
Percutaneous Interventions
Percutaneous interventions on the urinary tract came much earlier than the discovery of X-rays. In 1686, Toler inserted cannulas through the perineum to relieve urinary retention from impassable urethral strictures.
Riolan used a suprapubic approach to the bladder, while Heisler, in 1770, left a suprapubic cannula in place permanently in men suffering from bladder outlet obstruction.
In the latter half of the tenth century, the Arab physician Serapion is said to have thrust a red-hot iron through the flank and extracted a renal calculus. A related story is the one of Hobson, British consul at Venice in the mid-seventeenth century, who, following surgery for renal colic, continued to pass urine through a fistula in his flank until one day his wife, using a small dagger for a probe, extracted a date-shaped calculus from the tract, after which the man had no more symptoms [21].
Thomas Hiller, a British pediatrician, in 1864, inserted a needle into the hydronephrotic kidney of a four-year-old boy and removed more than three liters of urine, repeating the procedure several times during the boy’s life. [22] Several physicians took up this practice, but because of serious complications related to the procedure, especially peritonitis, caused it to be undertaken only under the most obliging circumstances, and it was eventually abandoned.
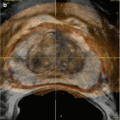
Interventional uroradiology as we know it today really began in 1939 when Archie Dean, an urologist at Memorial Hospital in New York, performed the first diagnostic percutaneous puncture of a renal mass. The return of clear fluid rather than blood made it possible to differentiate between cyst and neoplasms. However, in 1954, Wickbom, in Sweden [23], first utilized percutaneous puncture of the renal pelvis for antegrade pyelography and used this technique systematically in the diagnosis of outflow obstruction (Figs. 1.3 and 1.4).
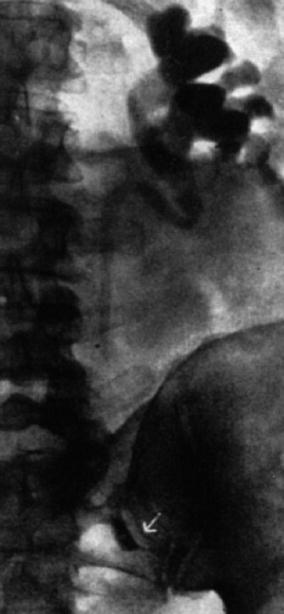
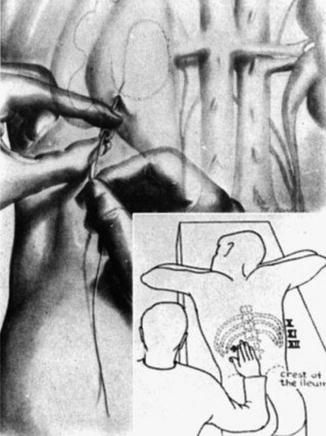

Fig. 1.3
Antegrade pyelography (1954). After direct puncture of the left renal pelvis, contrast material demonstrates dilation of the pelvis and upper part of the ureter. There is complete obstruction of the ureter at the pelvic inlet (arrow) (From Wickbom. [23] with permission)

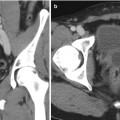
Fig. 1.4
Percutaneous trocar nephrostomy (1955): method and landmarks. Optimum puncture site is usually about five fingerbreadths lateral to midline and at a level where a 13th rib would be (From Wickbom. [23] with permission)
A year later, Goodwin and Casey, from the University of California in Los Angeles, were the first to use percutaneous nephrostomy as a therapeutic approach for draining obstructed kidneys and gaining surgical access to the renal collecting system, leaving a length of polyethylene tubing for drainage [24].
Kurt Lindblom reported percutaneous puncture of both cystic and solid renal masses employing, for the first time, fluoroscopy for localization and contrast material instillation to outline the interior of the lesion [25].
The utility of upper urinary tract access was further expanded when Kapandji performed manometric studies of the renal pelvis following percutaneous puncture, serving as a base for the work of Robert Whitaker of Cambridge, England, who perfected the technique of pyeloureteral infusion with pressure-flow monitoring [26].
Goodwin and Casey’s method was essentially blind, in order for them to insert a large trocar successfully, and it needed a markedly dilated renal collecting system. In 1965, Bartley and his associates in Göteborg in Sweden adapted Seldinger’s method of vascular catheterization and implemented it in the placement of percutaneous nephrostomies (Fig. 1.5). They described the use of fluoroscopic localization, guide wires, and angiographic catheters, thereby giving rise to percutaneous nephrostomy (PCN) as it is similarly performed today [28].

Fig. 1.5
Seldinger’s technique (From Seldinger. [27] with permission). 1 Needle inserted into a vessel. 2 Wire guide inserted through the needle. 3. Needle comes out, leaving the wire guide in place 4–6 Catheter inserted over the wire and into the vessel
In 1976, radiologist Ingmar Fernstrom and urologist Bengt Johansson published their benchmark work on removal of kidney and ureteral stones through a percutaneous approach and thus dramatically changed the practice of urology, giving birth to percutaneous nephrolithotomy (PCNL) [29].
Ultrasound
While at first renal biopsies were done “blindly,” in 1956, Lusted and his associates introduced biopsy under fluoroscopic control. Subsequently, almost every imaging modality came to be utilized in localizing the kidney for biopsy [30]. Sonography-guided localization, which was first suggested by Berlyne in 1961, became the most popular method until this day [31].
The human application of ultrasound began in 1880 with the work of brothers Pierre and Jacques Curie, who discovered that when pressure is applied to certain crystals, they generate electric voltage [32].
In 1912, the sinking of the RMS Titanic sparked the public’s desire for a device capable of echolocation. This was intensified 2 years later with the beginning of World War I, as submarine warfare became a vital part of war strategy. Canadian inventor Reginald Aubrey Fessenden—perhaps most famous for his work in pioneering radio broadcasting and developing the Niagara Falls power plant—volunteered during World War I to help create an acoustic-based system for echolocation. Within 3 months, he developed a high-power oscillator consisting of a 20 cm copper tube placed in a pattern of perpendicularly oriented magnetic fields that was capable of detecting an iceberg two miles away and being detected underwater by a receiver placed 50 miles away [33].
In 1936, German scientist Raimar Pohlman described an ultrasonic imaging method based on transmission via acoustic lenses, with conversion of the acoustic image into a visual entity. Two years later, Pohlman became the first to describe the use of ultrasound as a treatment modality when he observed its therapeutic effect when introduced into human tissues [34].
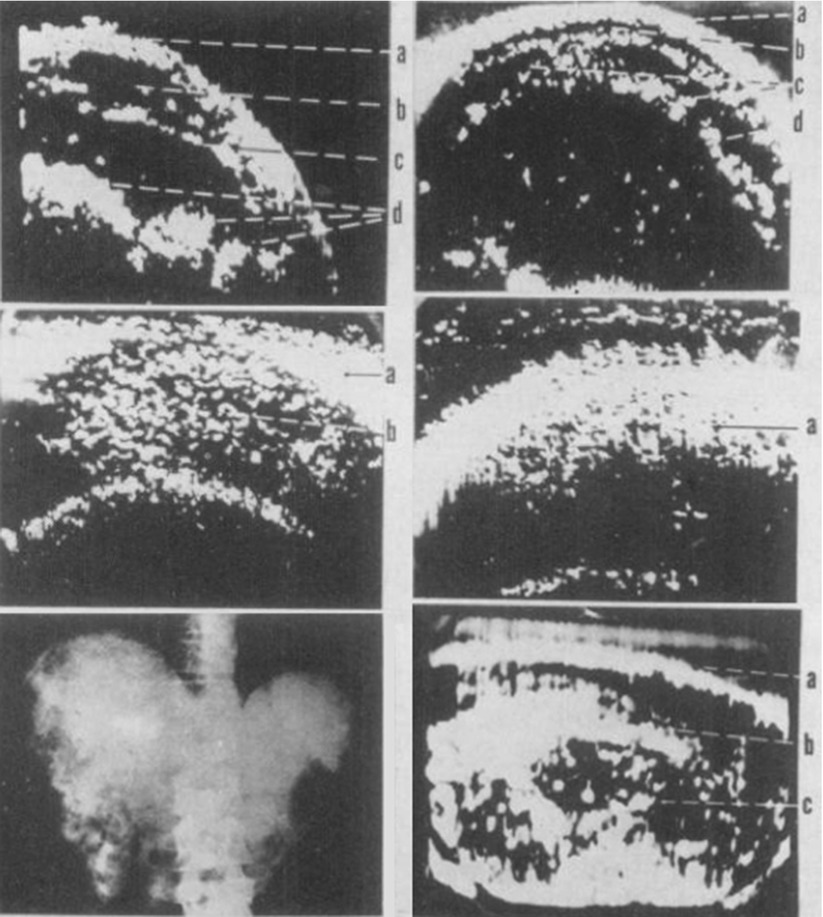
A few years later, in 1954, Dr. Joseph Holmes, a nephrologist, described the use of ultrasound to detect soft tissue structures with an ultrasonic “sonascope.” This consisted of a large water bath in which the patient would sit, a sound generator mounted on the tub, and an oscilloscope which would display the images. The sonascope was capable of identifying a cirrhotic liver, renal cyst, and differentiating veins, arteries, and nerves in the neck [35] (Fig. 1.6).
Get Clinical Tree app for offline access

Fig. 1.6
Series of somagrams taken over the liver area of the abdomen. The first is of a normal individual as food is passing down the intestinal tract. (a) abdominal wall, (b, c) intestinal tract, (d) food. The second is of a patient with moderately advanced cirrhosis with hepatomegaly. (a, b) abdominal wall, c ascites, d




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree