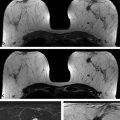
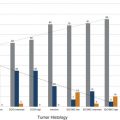
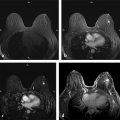
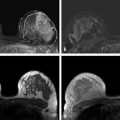
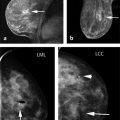
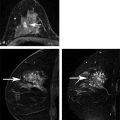
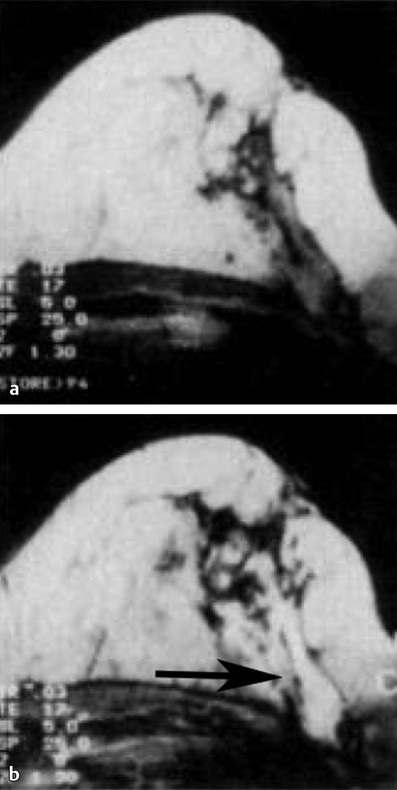
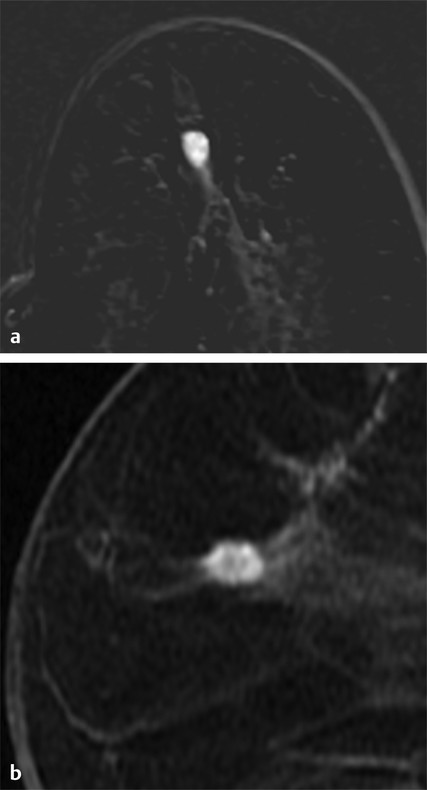
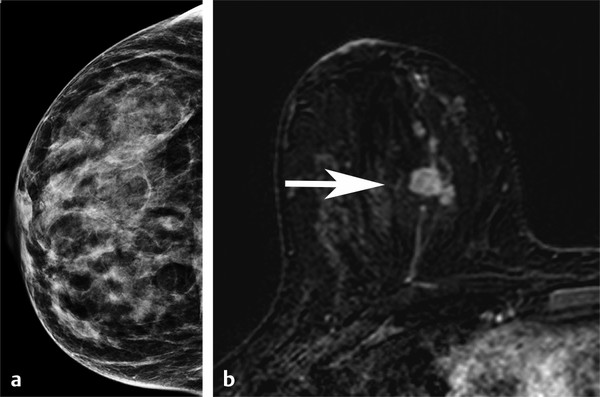
Importance of Early Detection: What Have We Learned from Mammography? Multiple randomized controlled trials and observational and service studies in Europe and North America have demonstrated that breast cancer mortality decreases by approximately 30% once mammographic screening is instituted, suggesting that the early detection of breast cancers before they are clinically apparent reduces deaths due to breast cancer. 2, 3, 4, 10 Meta-analyses have confirmed this reduction in breast cancer mortality begins 5 to 7 years after the institution of mammographic screening programs. 11 In addition to reducing mortality, early detection through breast cancer screening also results in less invasive and aggressive therapy. The Swedish trials demonstrated that screening mammography detects small, node-negative breast cancers, allowing patients to receive less aggressive surgical as well as adjuvant therapy. 12 Therefore, early breast cancer detection through mammographic screening programs both reduces breast cancer morality and improves treatment options. Although mammography is an effective screening test, the limitations of mammography discussed earlier, particularly in high-risk women and in women with dense breasts, have led to interest in other modalities to supplement mammographic screening. Any alternative or supplemental screening test has been measured by its ability to detect small node-negative breast cancers. Research in the use of breast MRI began in the 1980s in the United States and Germany. Breast MRI was initially performed in the early 1980s using a body coil, but dedicated breast coils were soon developed by the mid-1980s. 13, 14, 15 In 1986, Heywang et al demonstrated that cancers on breast MRIs performed following gadolinium administration demonstrated increased enhancement relative to the normal breast parenchyma, findings confirmed on subsequent studies ( ▶ Fig. 2.1). 16, 17 Most breast cancers demonstrate early enhancement (rapid wash), while the normal breast parenchyma progressively enhances with time. Therefore, neoplasms are most discernible in the early postcontrast phase, approximately 60 to 120 seconds after contrast injection. During the 1990s, efforts were directed at improving image acquisition and interpretation. Much debate centered on whether the emphasis should be on enhancement kinetics or lesion morphology. Was it more important to detect a washout kinetic pattern indicating possible angiogenesis associated with invasive cancer or obtain detailed information on morphology to distinguish a benign from malignant lesion? High temporal resolution implies rapid image acquisition in order to maximize information on lesion kinetics, while high spatial resolution imaging obtains thin slices in order to maximize information on lesion morphology. High spatial resolution images (slice thickness ≤3 mm and <1 mm in plane resolution) are necessary to both detect small lesions and assess their morphology ( ▶ Fig. 2.2). Therefore, improving spatial resolution and increasing temporal resolution represent competing interests. Any increase in spatial resolution requires an increase in acquisition time. Reducing temporal resolution decreases sampling rate, which may lead to a loss in the ability to detect changes in signal intensity. Kuhl et al examined the trade-off between spatial and temporal resolution. Although there is a loss of kinetic information when reducing temporal resolution and increasing spatial resolution, kinetic information is usually preserved with acquisition times of 2 minutes or less due to the broad overlap of enhancement rates between benign and malignant lesions, with the benefit of gaining improved morphological information. 18 Current full breast MRI protocols are performed on either 1.5- or 3-T magnets and provide both high spatial resolution (≤3 mm slices with ≤1 mm in-plane spatial resolution) and high temporal resolution (acquisition time within ≤2 min). Fig. 2.1 Early breast magnetic resonance imaging (MRI). Axial images of an enhancing breast cancer (black arrow) on an early breast MRI performed in 1989 (a) before and (b) after intravenous gadolinium injection. (Adapted with permission from Kaiser and Zeitler 17.) Fig. 2.2 Importance of high spatial resolution. (a) Axial subtracted image from a low spatial resolution magnetic resonance imaging (MRI) demonstrates what appears to be a circumscribed homogeneously enhancing mass with nonenhancing internal septations, suggestive of a fibroadenoma. (b) The same lesion imaged at high resolution demonstrates spiculated, irregular rim enhancing mass. Biopsy yielded invasive ductal carcinoma. Initial studies reported a very high sensitivity of breast MRI for invasive cancers, spurring interest in the possibility of using MRI as a supplemental screening modality. 17, 19, 20 Kuhl et al published the first study on using breast MRI to screen women at high risk for breast cancer. 21 This study included 192 women with a suspected or known carrier of a breast cancer susceptibility gene. In that study, six of the nine cancers diagnosed were seen only on MRI and occult on both mammography and ultrasound, demonstrating a significantly higher accuracy of breast MRI compared to conventional imaging when used to screen high-risk women. In 2004, Kriege et al published their results from the Netherlands comparing the sensitivity of MRI to mammography and clinical breast exam in one of the largest screening MRI trials, which included 1,909 women. In this study, the sensitivity of MRI was 79.5% compared to 17.9% for clinical breast exam and 33% for mammography. 22 Multiple subsequent studies confirmed the substantially higher sensitivity of breast MRI for invasive cancers compared to both mammography and ultrasound, resulting in current recommendations of annual screening MRI as an adjunct to annual mammography in women at high risk for breast cancer ( ▶ Fig. 2.3; ▶ Table 2.1). 22, 23, 24, 25, 26, 27, 28 Supplemental screening with breast MRI is recommended by the American Cancer Society (ACS), the National Comprehensive Cancer Network (NCCN), and joint recommendations from the Society of Breast Imaging (SBI) and the American College of Radiology (ACR) for patients with a known BRCA1 or BRCA2 mutation and for their untested first-degree relatives, for women with a lifetime risk of 20 to 25% or greater, and for women with a history of chest irradiation. 29, 30 Annual screening with MRI and mammography is also recommended for women with Li–Fraumeni syndrome, Cowden syndrome, or Bannayan–Riley–Ruvalcaba syndrome. Fig. 2.3 Mammographically occult breast cancer. (a) Right craniocaudal (CC) view from a digital breast tomosynthesis study demonstrates no abnormality. (b) Axial subtracted image demonstrates an irregular heterogeneously enhancing mass (white arrow) in the medial right breast. Biopsy yielded invasive ductal carcinoma. Author (year) CA/No. of women (%) Sensitivity Specificity MG (%) US (%) MR (%) MG (%) US (%) MR (%) Kriege et al (2004) 22 50/1,909 (3) 40 – 71 95 – 90 Warner et al (2004) 28 22/236 (9) 36 33 77 100 96 95 Kuhl et al (2005) 23 43/529 (8) 33 40 91 97 91 97 Leach et al (2005) 24 35/649 (5) 40 – 77 93 – 81 Hagen et al (2007) 25 25/491 (5) 50 – 86 – – – Rijnsburger et al (2010) 26 97/2,157 (4) 41 – 71 95 – 90 Sardanelli et al (2011) 27 52/501 (10) 50 52 91 99 98 97 Abbreviations: CA, cancer; MG, mammography; MRI, magnetic resonance imaging; US, ultrasound. Genetic abnormalities account for 5 to 10% of all cases of breast cancer. 31 The most well-known are mutations involving the BRCA1 and BRCA2 genes, identified in the 1990s. 32 The prevalence of a BRCA mutation is approximately 1/500 to 1/1,000 in the general population and 1/50 in women with a Jewish ancestry. The estimated risk for breast cancer by the age of 70 ranges from 46 to 87% with a BRCA1 mutation and 37 to 84% with a BRCA2 mutation. 33 The BRCA1 gene, located on chromosome 17, is a tumor suppressor gene. The BRCA1 gene is thought to be responsible for the breast/ovarian syndrome, and accounts for 50% of familial breast cancers and 5 to 8% of all breast cancers. The relative risk associated with a BRCA1 mutation declines with advancing age. The BRCA2 gene is located on chromosome 13 and accounts for approximately 35% of familial breast cancers. BRCA2 carriers are at risk for developing other cancers, including prostate, bladder, pancreatic, and Hodgkin’s disease. Mutations in other genes, such as the TP53 and PTEN genes among others, are also known to confer a high risk for breast cancer but are much less common. 34 There has been increasing recognition of the role of tumor biology in BRCA1 and BRCA2 mutation carriers. BRCA1-related breast cancers differ from sporadic cancers in that they are often of higher histological grade, show lymphocytic infiltration, and are more likely to be aneuploid and triple negative (estrogen, progesterone, and human epidermal growth factor 2 receptor negative). 35, 36 The interval cancer rate, that is, cancers detected in between annual screening, is also known to be high in BRCA mutation carriers, due to the rapid tumor growth rate in these women. 9 Studies have also shown that in BRCA1 or BRCA2 mutation carriers, the tumor growth rate is double that of nonmutation carriers and is highest in women who develop breast cancer at the youngest ages. 37 Despite the widespread evidence that mammographic screening improves mortality in the general population, there is little evidence that mammographic screening reduces cancer mortality in high-risk mutation carriers. Evans et al compared survival rates in women with a likely or known BRCA1, BRCA2, or TP53 mutation that were not being screened for breast cancer, screened with mammography alone, or screened with a combination of mammography and MR. 38 There was a significant survival benefit for those women undergoing surveillance compared to those not screened, and a borderline significant advantage in survival between those screened with a combination of mammography and MRI compared to those screened with mammography alone. Multiple retrospective and prospective studies have demonstrated the limitations of mammography in high-risk patients, especially in those with a BRCA mutation. The sensitivity of mammography in young, high-risk women with dense breast tissue is consistently reported to be less than 50%. In addition, of the cancers detected, 40 to 78% of the invasive cancers are greater than 1 cm in diameter and axillary nodes are involved in 20 to 56%. 7, 8, 9 Explanations for the low sensitivity of mammography in these studies may include the higher breast density in younger women, a higher likelihood of rapidly growing tumors, and the more benign appearance of many BRCA1 cancers, making them more difficult to detect. 6 Only 1 to 2% of women have a family history suggestive of an autosomal dominant gene mutation. Although the majority of women in the general population have at least one relative with a history of breast cancer, this does not confer an increased risk because that cancer was sporadic or confers a low increase in risk due to a low penetrance gene. Features that suggest that the breast cancer may be due to a high penetrance gene include two or more close relatives with breast or ovarian cancer, breast cancer occurring before age 50 in a close relative, a family history of both breast and ovarian cancer, one or more relatives with two cancers (breast and ovarian cancer or two independent breast cancers), and male relatives with breast cancer. Supplemental screening with breast MRI is also recommended for women with a lifetime breast cancer risk of greater than 20 to 25%. Multiple models are available to assign risk, including Gail, Claus, Tyrer-Cuzick, Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), and BRCAPRO models. 39, 40, 41, 42, 43 Each of these risk assessment models incorporates different risk factors to calculate the breast cancer risk. The Gail model does not factor in a family history other than a first-degree relative and is specifically not recommended to be used to determine whether supplemental screening with MRI is indicated. 44 The Tyrer-Cuzick model includes both family history and a history of high-risk lesions such as lobular carcinoma in situ (LCIS) and atypical ductal hyperplasia (ADH), which are not included in the other risk assessment models. 45 Because each model incorporates different risk factors, the calculated lifetime risk may vary widely depending on the model used. For example, the lifetime risk of a 35-year-old woman with a mother diagnosed with breast cancer at the age of 51 and a maternal aunt diagnosed with breast cancer at the age of 60 would have a BRACAPRO lifetime risk of 13%, a Claus risk of 18%, and a Tyrer-Cuzick risk of 23%. 46 As discussed earlier, starting from the mid-1990s, multiple prospective studies were performed to evaluate the utility of MRI as an adjunctive screening tool in women with a known or likely genetic mutation predisposing them to breast cancer. 22, 23, 24, 28, 47, 48, 49, 50 The risk factors of the women included in these studies vary widely, with some studies including only women with a known or high suspicion for a BRCA mutation and others including women with a wide range of risk factors including a strong family history or personal history of breast cancer or prior biopsy demonstrating LCIS or ADH. Despite these differences, the sensitivity of breast MRI in all these studies was significantly higher than that of mammography, with a sensitivity of approximately 90%. In these trials, the sensitivity of MRI ranged from 71 to 100%, compared to 13 to 59% for mammography. When data from 11 studies were combined in a meta-analysis, it was found that there was a sensitivity of 77% for MRI alone, 94% for a combination of MRI and mammography, and 39% for mammography alone. 51 Therefore, the highest sensitivity was achieved using a combination of mammography and MRI. The addition of ultrasound has not been shown to improve cancer detection rates beyond that achieved with MRI and mammography. 52 The prevalence of mammographically occult breast cancer in the first round of screening MRI is extremely high at between 1.1 and 10%, which is about 10 times the expected rate in the general population. 53 In studies where more than one round of MRI screening was performed, the incidence was found to be between 0.6 and 4%, indicating a continued benefit to MRI screening in this population after the first screening round. While some of these studies include women with a “strong family history” of breast cancer or other risk factors, it is not surprising that the highest cancer detection rates were reported in those studies evaluating patients with BRCA1 and BRCA2 mutations.
2.3 Early Research on Breast MRI
2.4 MRI for High-Risk Screening
2.4.1 Hereditary/Familial Risk of Breast Cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree